How to optimize anti-infective treatment strategies for pneumonia?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
How to optimize anti-infective treatment strategies for pneumonia?
How to optimize anti-infective treatment strategies for pneumonia? Pneumonia is the third leading cause of death worldwide, and approximately 50% of sepsis and septic shock are caused by pneumonia.

1. Summary
1) In the era of the proliferation of multi-drug resistant bacteria, clinicians should determine the dose of antibiotics based on pharmacokinetics and pharmacodynamics (PK/PD), so as to maximize the anti-infective effects of antibiotics, reduce toxic side effects Resistance.
2) The currently recommended anti-infection strategy cannot adapt to all patients and all pneumonia.
3) PK/PD is a core content in the development of antibiotics, and it is very important.
4) Atomized administration of antibiotics can make antibiotics act on the respiratory tract quickly and can be cleared quickly, thereby reducing drug resistance and systemic reactions.
5) The combination drug has a certain synergistic effect, but it is still necessary to further clarify whether the in vitro test and the clinical response are consistent.
6) There is currently no evidence to support the monitoring of antibiotic strategies, and there is a lack of consistency in monitoring methods between hospitals. Therefore, standardized guidelines are needed to guide related treatment.
2. Introduction
Pneumonia is the third leading cause of death worldwide, and approximately 50% of sepsis and septic shock are caused by pneumonia. Community-acquired bacterial pneumonia (CABP) and hospital-acquired bacterial pneumonia (HABP) have high morbidity, mortality, long hospital stays and high costs. Ventilator-related bacterial pneumonia (VABP) can prolong hospital stay and ICU time, increase mechanical ventilation time, and increase mortality. According to statistics, in 2013, the entire United States spent 9.5 billion on pneumonia. Although the current anti-infective strategies are constantly improving, the probability of HABP and CABP treatment failure is still high, 30-62% and 2.4-31%, respectively.
There are many factors that can lead to failure of the treatment of pneumonia, among which the choice of anti-infection strategies is an important aspect. The most important point is that the selected antibiotics need to be effective against potential bacteria. However, the emergence of multi-drug resistant bacteria (MDR) and pan-drug resistant bacteria (XDR) makes anti-infective treatment very difficult. Ensuring the effectiveness of anti-infection and avoiding inappropriate use of spectrum antibiotics are clinically difficult to operate, and it is difficult to choose between the two.
ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter) bacteria account for 80% of HABP and are often resistant.
[Editor’s note: Enterobacteriaceae contains many types of medical-related bacteria, which can parasitize in the human intestine. Many bacteria are normal flora and conditional pathogenic bacteria, which can cause intestinal and extraintestinal infections. Among them, plague Yersinia and Salmonella typhi are very pathogenic to humans. Escherichia is mainly Escherichia coli; Shigella includes Shigella dysenteriae, Shigella flexneri and so on. Enterobacter is a normal intestinal bacterium, which can cause conditional disease, and the treatment process is easy to cause drug resistance, including: Enterobacter cloacae, Enterobacter aerogenes, Enterobacter Tyrolus, Enterobacter sakazakii]
While choosing the right antibiotic, you also need to choose the right dose. Many antibiotics lack indications for the treatment of pneumonia. The dose of antibiotics used to treat infections in other areas may not reach the required concentration in the lungs. And there are differences between different patients, such as obese patients, critically ill patients, patients with increased kidney clearance, patients receiving organ transplants, patients receiving dialysis or ECMO, etc. In these patients, the drug concentration will change, so the drug The dose needs to be adjusted.
The best anti-infective strategy includes selecting the appropriate antibiotics, using the appropriate route of administration, using the appropriate drug dosage, and anti-infective time. This optimized anti-infection strategy can effectively improve the prognosis of patients with pneumonia. The ideal anti-infection requires maximizing the response of microorganisms to antibiotics while reducing drug toxins and avoiding drug resistance. This article will discuss the appropriate anti-infection strategies for pneumonia based on the PK/PD theory.
How PK/PD research determines the best drug dosage and personalized treatment in the clinic. IDSA/ATS recommends using PK/PD to determine the drug dose when treating HABP/VABP, but the recommendation level is weak recommendation. PK/PD guides the clinical application of antibiotics. Although the shortcomings of this method are not clear enough, it does increase the medical expenses and burdens of patients. There is no clinical evidence to support it, so the current recommendation level is weak. In the current environment, PK/PD-guided personalized antibiotic adjustment should be recommended, although whether it can improve the prognosis still needs further discussion.
In the era of the proliferation of multi-drug resistant bacteria, it is vital to ensure the effectiveness of anti-infection. Although some new drugs are currently under development, some drugs lack clear indications, and some drugs cannot be used in specific populations. Individualized treatments are required for different patients, which makes the PK/PD perspective indispensable. This article emphasizes the clinical view of pharmacokinetics and pharmacodynamics, focusing on how to carry out the best anti-infective treatment.
3. PK/PD optimization principle
With the increasing number of drug-resistant bacteria, the correct use of antibiotics is now strongly emphasized. The correct use of antibiotics is based on the pharmacological characteristics of antibiotics. Pharmacokinetics (PK) is a branch of pharmacology that studies the metabolism of drugs in the human body, the concentration in different tissues of the human body, and the human body’s response to drugs. Pharmacokinetics can help establish the quantitative relationship between the dose and the blood drug concentration, which is very important because the drug concentration will determine the prognosis of the patient.
The PK model is based on the concept of “room”. The one-compartment model theory has only one central compartment, such as plasma. The multi-compartment model contains a central compartment and a peripheral compartment. The peripheral compartment can be different distant tissues, such as fat and lungs. At the same time, the pharmacokinetic model can also treat the patient as a whole without using the “chamber” model.
The concentration-time curve of the drug reflects the absorption, distribution, metabolism and clearance of the drug. “Absorption” describes the rate at which the drug enters the central compartment after administration, such as the rate at which the gastrointestinal tract absorbs the drug into the blood after oral administration. “Distribution” determines the transfer and distribution of drugs between various “rooms”. This process is dynamic and two-way. “Metabolism” describes the transformation and deformation of drugs, and “clearance” describes the process by which drugs are transferred out of the body.
Pharmacokinetics can also be affected by the biochemical efficacy of the drug, such as the solubility and water solubility of the drug. Hydrophilic antibiotics can be distributed in intravascular and extravascular tissues, while lipophilic antibiotics can be distributed in adipose tissue and cells.
Another factor affecting the pharmacokinetic characteristic curve is the protein binding rate of the drug. The drugs bound to the protein cannot be transferred from the central compartment to the peripheral compartment and cannot react with other molecules. Therefore, for antibiotics, only the antibiotics that are not bound to the protein have anti-infective effects.
Generally speaking, the more acidic and normal PH drugs are easily bound to albumin, and the more basic ones are bound to α-1 acid glycoprotein. Changes in protein levels, such as hypoalbuminemia, can change the free concentration of antibiotics.
Pharmacokinetic models can help understand the distribution characteristics of drugs. Drug clearance (Clear, CL) is a parameter of pharmacokinetics, studying the metabolism and clearance of drugs, that is, the changes in the plasma concentration of drugs per unit time. CL can be personalized according to different organs, just add the corresponding organ name abbreviation after CL, such as kidney CL (CLR), liver CLH. The volume of distribution (VD) is another parameter of pharmacokinetics, which represents the theoretical volume of drug distribution.
For example, if an intravenous administration of 100 mg reaches a blood concentration of 0.005 mg/ml, the volume of distribution is 20L. The transfer of the drug from the central chamber to the peripheral chamber increases the volume of distribution. These parameters are both affected by the characteristics of the body and the drug. These parameters can help understand the plasma concentration and tissue concentration after antibiotic application, and provide a pharmacokinetic curve.
Pharmacokinetic curve
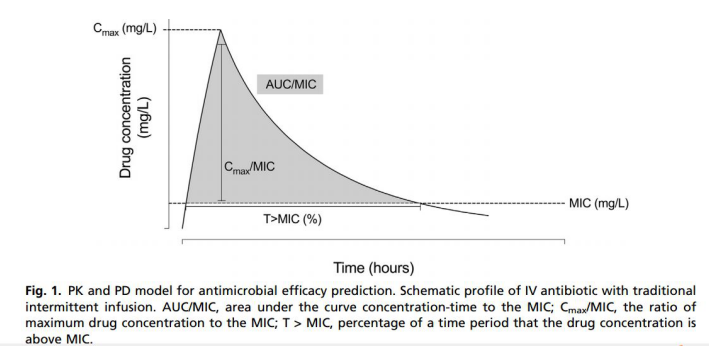
Another branch of pharmacology is pharmacodynamics (PD), which studies the effects of drugs after entering the human body, that is, what changes the drugs can bring and what effects they play. The therapeutic effect of antibiotics depends on the specific point in the concentration-time curve where the antibiotic works, and it is also affected by the sensitivity of microorganisms to antibiotics, which is the minimum inhibitory concentration (MIC). In vitro models have clarified the effects of antibiotics and pathogenic bacteria in vitro, but whether the body can successfully fight infection still depends on toxicity, immune response and the site of infection. Although some patients still cannot control the infection after antibiotics, the use of pharmacodynamics to guide the application of antibiotics can still bring some benefits.
According to pharmacodynamics, antibiotics are divided into three categories: concentration-dependent, time-dependent, and mixed. For concentration-dependent antibiotics, the higher the concentration, the better the effect. Such antibiotics should be given at a high concentration and the interval between administrations should be longer. The effect of time-dependent antibiotics depends on the time when the drug concentration is above the MIC.
A high concentration does not have any better benefit for this type of antibiotic. This type of antibiotic should be continuously infused, or a single dose of small, repeated administration Administration. For comprehensive antibiotics, increasing the time and concentration will not have a better effect. The effect of these antibiotics depends on the area under the curve (AUC). For time-dependent antibiotics, you need to pay attention to the time when the concentration exceeds MIC, that is, fT> MIC. For concentration-dependent antibiotics, you need to pay attention to the ratio of the highest free concentration to MIC, that is, fCmax/MIC. For mixed antibiotics, you need to study MIC.
The area under the upper curve is fAUC24h/MIC. It should be noted that these parameters are studied for free antibiotics, not antibiotics bound to proteins, because antibiotics bound to proteins have no effect. For β-lactam and macrolide antibiotics, fT>MIC can predict the anti-infective effect. The fCmax/MIC index is suitable for aminoglycosides, quinolones, and colistin, while the fAUC/MIC index is suitable for Vancomycin, oxazolidinone antibiotics.
Pharmacodynamics also needs to consider the post-antibiotic effect (PAE). This phenomenon was described when penicillin was discovered to describe the restriction of bacterial growth after the application of antibiotics. For example, after the antibiotic is completely metabolized, the bacteria still cannot grow normally. The PAE effect of aminoglycoside antibiotics is the most significant, which may be irreversibly bound to the ribosome of the bacteria with this type of drug, thereby playing a PAE effect. For β-lactam drugs, except for carbapenems, there is no post-antibiotic effect when treating gram-negative bacteria, but when treating gram-positive bacteria, there are reports that they have a post-antibiotic effect.
Classification of antibiotics
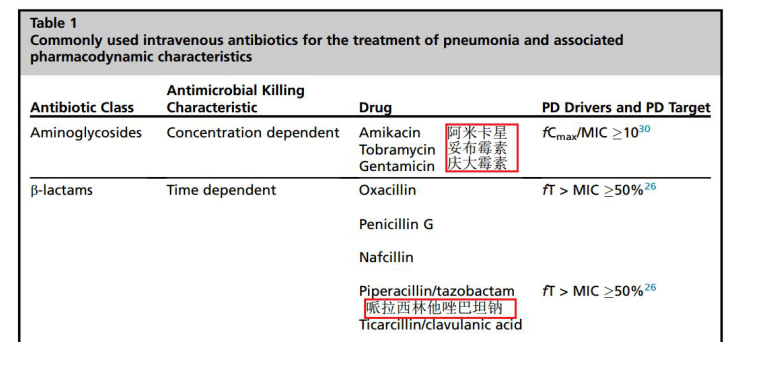
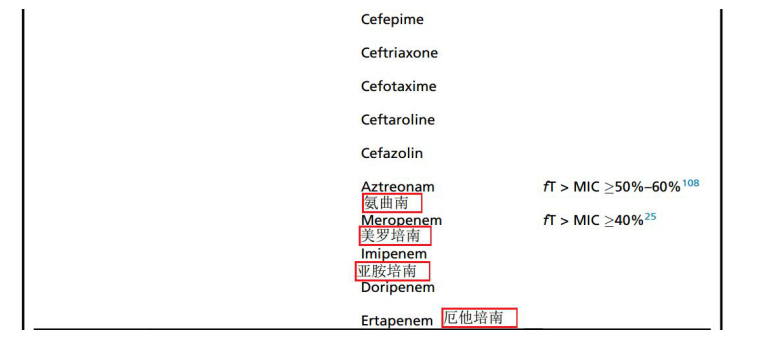
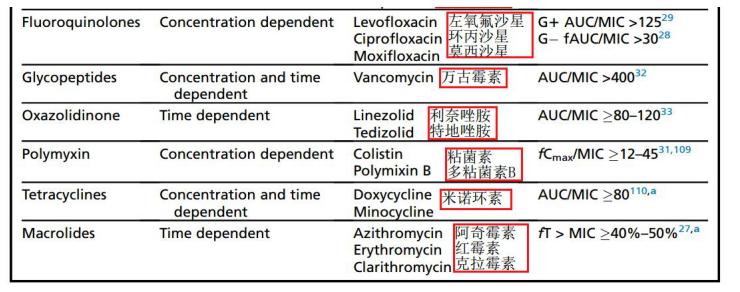
4. Precautions for pneumonia patients and critically ill patients
Measuring the blood concentration of antibiotics can provide important information. If the MIC value of the isolated antibiotic flora can be obtained at the same time, the clinician’s anti-infection strategy will become more justified. Unbound antibiotics can penetrate through the capillaries to reach the interstitial spaces, and the plasma concentration can reflect the tissue concentration. However, sometimes the blood drug concentration cannot reflect the tissue concentration.
The lung tissue drug distribution is quite special and cannot be reflected based on the plasma pharmacokinetic value. The lung tissue itself contains multiple “compartments”, such as alveolar epithelial cells, endothelial cells, lung interstitium, and alveolar macrophages. Therefore, for the lungs, there are many factors that can affect the penetration of the drug and the concentration of the drug to the infected site. First of all, only unconjugated antibiotics can penetrate the blood barrier and reach the inside of the lung tissue.
Therefore, for the lung, the drug diffusion speed refers to the speed at which the drug penetrates the lung. This speed is affected by the lipophilicity, polarity, and diffusivity of the drug, which can be explained by Fick’s theory.
As mentioned earlier, lipophilic, small-molecule antibiotics such as linezolid, can penetrate lung tissue easily compared to hydrophilic antibiotics such as vancomycin. Host factors can also affect the penetration of drugs into lung tissue, such as inflammation, pH, and so on. Therefore, the plasma drug concentration will be different from the local tissue concentration. Also, even for the same lung, the tissue concentration is different among diseased and healthy people, indicating that the lung is a heterogeneous tissue, especially when the lung is infected, the drug concentration in the lung tissue can be in the lung tissue. Fluctuations in different parts.
In view of the above, the drug concentration of alveolar epithelial cell fluid (Epithelial lining fluid, ELF) can better reflect the penetration of the drug into the lung tissue. ELF can be measured by bronchoalveolar lavage fluid. Since bronchoalveolar lavage will dilute the alveolar fluid, it can be corrected by the urea concentration of the lavage fluid and the urea concentration of the blood, because urea can freely pass through the blood and Alveoli. VELF = VBAL * UreaBAL/Urea serum. This technique has some limitations.
When performing fluid correction, it is necessary to assume that antibiotics and urea can penetrate the lungs and reach the lung tissues, but for the case of low penetration, the data may not be accurate enough. ELF concentration is only suitable for assessing the exposure of extracellular antibiotics. If alveolar macrophages are decomposed, antibiotics may be released, which can affect the measured value.
The drug concentration-time curve of ELF and plasma drug concentration-time curve may be inconsistent. For some antibiotics, there is a linear relationship between the lung concentration and the plasma concentration. At this time, the concentration-time curve is parallel, such as macrolides, oxazolidinones, and fluoroquinolones, and the ELF concentration will be higher than the plasma concentration .
Beta lactam antibiotics, glycopeptide antibiotics and aminoglycoside antibiotics can also show this relationship, but the ELF concentration is usually lower than the plasma concentration. On the contrary, other antibiotics such as carbapenems, ELF and plasma concentration are inconsistent, can show their own changes, for example, ELF concentration increases when the plasma concentration decreases, and vice versa, so the plasma concentration cannot reflect the lung tissue The current clinical research uses the area under the curve to describe the lung penetration of this drug, which is AUC plasma/AUC elf.
Some indicators of pharmacokinetics, such as CL and Vd, can be greatly affected in patients with pneumonia, especially in severely ill patients. For hydrophilic drugs, the impact is particularly large, because they are mainly distributed in the central compartment and outside the cell, and are also affected by renal function. For hydrophilic drugs, the volume of distribution is almost the same as the extracellular volume. In critically ill patients, the volume of distribution can double. In patients with sepsis, vasodilation and increased vascular permeability can lead to capillary leakage, resulting in fluid leakage, tissue edema and hypotension. Rehydration can be used to maintain blood pressure. At this time, the volume of distribution will increase, resulting in a decrease in the concentration of antibiotics. Other conditions, such as continuous drainage of surgical drainage tubes, mechanical ventilation, extracorporeal circulation, etc., can also cause the volume of distribution to increase.
Most of the critically ill patients will decrease albumin (<25g/L). Therefore, for antibiotics with high protein binding rate, because albumin decreases and binding sites decrease, the free antibiotics will increase. The higher the protein binding rate, the more antibiotics will be released during hypoalbuminemia. This will affect the pharmacodynamic effect, but it will also increase the drug distribution in the central compartment and increase the volume of distribution of antibiotics. Hypoproteinemia will also affect the clearance of antibiotics, because antibiotics that bind to proteins cannot pass through the glomerulus. Therefore, antibiotics with high protein binding rate will clear more when hypoalbuminemia. Some studies have shown that when hypoalbuminemia, antibiotics have a larger volume of distribution and increased clearance, which will lead to a decrease in the value of fT>MIC, resulting in poor anti-infective effects of time-dependent antibiotics, especially for high protein binding rates Of antibiotics, such as ertapenem and ceftriaxone.
The increased cardiac output of some critically ill patients leads to increased renal clearance (Augmented renal clearance, ARC), which will affect the effectiveness of anti-infective treatment. Usually, when the creatinine clearance rate exceeds 130 mL/min, it is considered that the renal function clearance rate is high, which will affect the clearance of hydrophilic antibiotics. For time-dependent antibiotics, such as β-lactam antibiotics, ARC will seriously affect the drug concentration, because such antibiotics need to ensure sufficient blood drug concentration. On the contrary, there are two cases of decreased clearance of drugs in clinical practice, namely acute kidney injury and liver insufficiency, which will increase the drug concentration.
ICU patients are more prone to infections with multi-drug resistant bacteria. Pneumonia mortality caused by multi-drug-resistant bacteria is higher, the length of ICU hospital stay is longer, and the prognosis is worse. These pathogenic bacteria have higher MIC values and require larger doses of drug concentration to achieve anti-infective effects. However, current research shows that when patients receive antibiotic treatment, the blood concentration is usually insufficient, not excessive.
5. Optimize the process
PK/PD is an important part of the clinical research and clinical use of antibiotics, but the current recommended amount of antibiotics often lacks individual differences among different patients. For example, β-lactam antibiotics need to be infused as soon as required, but extending the infusion time can increase the time of fT> MIC and improve the prognosis. It is also effective for hospital-acquired pneumonia. Some antibiotics have several dosage recommendations, and the dosage can be optimized using PK/PD theory. Many clinical trials require the use of PK/PD theory to guide clinical research, and the study of ceftazidime and avibactam is a famous example.
Ceftazidime Avibactam is a combination of β-lactam-β-lactamase, which can be used to treat infections caused by multi-drug resistant bacteria.
At the beginning, this drug was only used to treat abdominal infections and urinary system infections. The dose was 2.5g Q8H. Although it is recommended for the treatment of hospital-acquired pneumonia, PK was used to demonstrate its treatment of pneumonia. /PD theory. In vitro studies have shown that this drug is effective in treating Pseudomonas aeruginosa and Enterobacteriaceae, but it is not effective in treating Bowman immobility infection.
Ceftazidime is a β-lactam antibiotic and a time-dependent antibiotic. To use this antibiotic, fT> MIC must exceed 50% to be effective. This requires the establishment of the concentration-time curve relationship first, and then to explore the pharmacodynamics, to determine the dose and interval of administration. The final study showed that ceftazidime and avibactam administered at 2.5Q8H, each infusion for 2 hours, can achieve good therapeutic effects.
6. Local administration
Inhalation administration has been studied. It is a potential theoretical strategy for optimizing anti-infective therapy in the lungs, which can avoid the lack of systemic intravenous administration. Inhaled antibiotics can theoretically act directly on the respiratory tract, and can be quickly eliminated, reducing drug resistance and systemic exposure. Compared with previous drugs, the latest inhaled drugs can increase the accumulation of antibiotics in the lungs. Inhalation administration needs to consider the type, molecular weight, diameter, and airflow type of the inhaled drug.
Inhaled administration, especially aminoglycoside antibiotics, can produce potential therapeutic effects, but there are currently two clinical studies that have not found its benefits. At present, various studies have not shown that topical administration can improve the prognosis, and more and more detailed studies are needed to support this approach.
7. Combination medication and synergy
The combined use of two or more antibiotics to treat pneumonia is a standard practice, which can expand the antibacterial spectrum during the initial treatment period to cover common flora. Once the pathogen is clear, most patients’ anti-infective treatment needs to switch to monotherapy. However, the synergy between antibiotics can be used to increase the anti-infective effect. At this time, the combination therapy can be continued.
This method of treatment can increase the bactericidal effect and reduce drug resistance, especially for refractory bacterial infections, such as Pseudomonas aeruginosa. Combination medications usually use synergistic antibiotics. A classic example is β-lactam antibiotics combined with aminoglycosides to treat Gram-negative bacterial infections. Beta lactam antibiotics can destroy the cell wall, which can help aminoglycoside antibiotics to enter their site of action. In vitro studies have found that when treating Pseudomonas aeruginosa infections, cephalosporin antibiotics can be combined with fosfomycin, colistin, and amikacin.
However, this combination drug cannot be used routinely, and can only be used when the patient has a higher risk of death and the pathogens are resistant to strong bacteria. What needs to know is that the success of the in vitro test does not mean that the combination of drugs in vivo must be effective. At present, there are many inconsistent studies in in vivo and in vitro tests.
8. Therapeutic drug monitoring
Therapeutic drug monitoring (TDM) for individual patients can be used to adjust the drug dosage and achieve the appropriate drug concentration. At present, the concentration of aminoglycoside drugs and glycopeptide drugs is often monitored, because they are more toxic at higher concentrations. In the treatment of pneumonia, drug monitoring is especially important because both of them have poor lung penetration.
For aminoglycoside drugs, it is a concentration-dependent antibiotic and can be allowed to exist in high concentrations, but for patients with renal impairment, the dosing interval can be extended. Hartford provides a model diagram that can be used to challenge the use of aminoglycoside drugs. Studies have shown that this use can reduce nephrotoxicity while maintaining similar clinical responses. The monitoring of vancomycin can only monitor the trough concentration, which is closely related to the therapeutic effect and determines the value of AUC.
The current PK/PD theory can have different results between different patients, so TDM can be used to optimize the use of some antibiotics, especially the use of β-lactam antibiotics, because these drugs are often under-administered. Studies have shown that 74.2% of critically ill patients need to adjust the dose of β-lactam antibiotics, and most of them need to increase the drug dose. For burn patients, 60% do not achieve fT> MIC. [Editor’s note: Time-dependent antibiotics require more concentration monitoring]
9. Summary
With the increasing number of multi-drug resistant bacteria, it is very important to use PK/PD to guide the application of antibiotics. This article describes some principles to improve the success rate of pneumonia treatment, and proposes the principles to reduce drug resistance. At the same time, the article explains the importance of clinical and preclinical PK/PD. For pneumonia, PK/PD is very important. Without this theory, effective antibiotics in vitro may not achieve the expected results.
At the same time, PK/PD can also reduce the side effects of drugs, and achieve the best therapeutic effect with the smallest administration, so that complications can be avoided. Although PK/PD has been clinically used to guide treatment, such as prolonging the administration of β-lactam antibiotics and giving aminoglycoside drugs once a day, it still needs further promotion and improvement in the clinic.
(source:internet, reference only)
Disclaimer of medicaltrend.org



