Mesenchymal stem cells approved for treatment of sequelae of COVID-19 pneumonia in Japan
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Mesenchymal stem cells approved for treatment of sequelae of COVID-19 pneumonia in Japan
Mesenchymal stem cells approved for treatment of sequelae of COVID-19 pneumonia in Japan. Clinical research data shows that stem cell technology has become an effective way to tackle COVID-19 pneumonia.
According to foreign media reports [1], the Kyushu Special Committee of Regenerative Medicine in Japan approved a plan for the use of autologous fat-derived mesenchymal stem cells to treat the sequelae of COVID-19 pneumonia. This patented technology developed by the Korea Stem Cell Research Institute has passed the current regenerative medicine plan for the sequelae of COVID-19 pneumonia after 120 days of review by a committee composed of 16 experts in various fields, based on specific scientific evidence of treatment and clinical research The review will be launched in Japan at the end of December.
The director of the Stem Cell Research Institute, which developed this therapy, said: “This approval has been strongly supported by the Special Committee of Regenerative Medicine. It is believed that mesenchymal stem cells will have a good effect in treating the sequelae of COVID-19 pneumonia through anti-inflammatory and tissue regeneration effects.”
It is reported that the COVID-19 pneumonia has spread on a large scale globally. With the increase of patients around the world, the risk has increased rapidly, and the third wave of pandemic has begun. Some patients with new coronavirus infection can cause severe sequelae even after treatment, such as brain fog, difficulty breathing and chest pain. According to an article published in the Journal of American Medical Association in 2020, 87.4% of COVID-19 patients were confirmed to have at least one sequelae effect, many of which were fatigue (53.1%), dyspnea (43.3%) and chest pain (21.7%) [1].
https://jamanetwork.com/journals/jama/fullarticle/2768351
Since patients with COVID-19 pneumonia were successfully treated, the World Health Organization has been deeply concerned about the long-term damage and long-term sequelae caused by COVID-19 pneumonia to the human body. Even after recovery, patients with COVID-19 pneumonia still have a continuous inflammatory response in their bodies. This has a great impact on the prognosis and recovery of patients with COVID-19 pneumonia.
Mesenchymal stem cells treat COVID-19 pneumonia and its sequelae
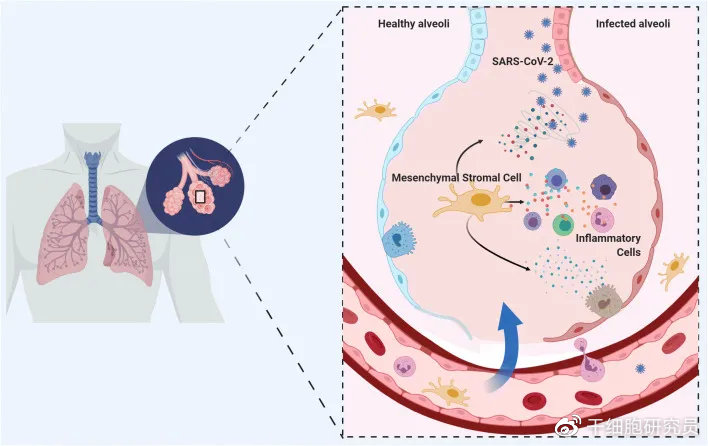
Mesenchymal stem cells have been shown to be effective in cell regeneration and reducing inflammatory factors in the body. Mesenchymal stem cells are adult stem cells with multidirectional differentiation potential. They can not only promote the regeneration and repair of damaged tissues, but also have good immune regulation capabilities. They can regulate inflammation by regulating the proliferation, differentiation and functional status of immune cells. Factor levels have good application prospects for various inflammation-related diseases [2]. Since the beginning of this year, domestic and foreign research results have been output in the field of stem cell treatment of COVID-19 pneumonia and its sequelae.
Picture from literature [3]
According to the literature [3], 7 patients with COVID-19 pneumonia (2 mild, 4 severe, and 1 critical) recruited by Beijing You’an Hospital in China received a single intravenous mesenchymal stem cell treatment. The results showed that within 14 days of transplantation , Mesenchymal stem cell therapy significantly improved the lung function of 7 patients, and there were no obvious adverse reactions. It is worth mentioning that within 2 days after mesenchymal stem cell treatment, all patients’ lung function and symptoms improved significantly. Within 2-4 days after treatment, all patients’ symptoms included: high fever, weakness, shortness of breath, and low blood oxygen saturation All disappeared, and the resting blood oxygen saturation was ≥95%. Two patients with mild illness and one patient with severe illness recovered and were discharged from the hospital within 10 days after treatment.
Internationally, recent data from the UAE Abu Dhabi Stem Cell Center (ADSCC) show that their stem cell therapy (UAECell19) has helped 5,000 patients recover from the new coronavirus infection. After the experiment, the researchers concluded that compared with patients receiving standard treatment, stem cell therapy (UAECell19) can shorten the hospital stay from 22 days to 6 days. Further analysis showed that patients receiving stem cell therapy were 3.1 times more likely to recover within 7 days than patients receiving standard treatment. Among patients receiving stem cell therapy, 67% of patients attributed their recovery to the new therapy.
Mesenchymal stem cells approved for treatment of sequelae of COVID-19 pneumonia in Japan
Mesenchymal stem cells approved for treatment of sequelae of COVID-19 pneumonia in Japan. Clinical research data shows that stem cell technology has become an effective way to tackle COVID-19 pneumonia.
According to foreign media reports [1], the Kyushu Special Committee of Regenerative Medicine in Japan approved a plan for the use of autologous fat-derived mesenchymal stem cells to treat the sequelae of COVID-19 pneumonia. This patented technology developed by the Korea Stem Cell Research Institute has passed the current regenerative medicine plan for the sequelae of COVID-19 pneumonia after 120 days of review by a committee composed of 16 experts in various fields, based on specific scientific evidence of treatment and clinical research The review will be launched in Japan at the end of December.

The director of the Stem Cell Research Institute, which developed this therapy, said: “This approval has been strongly supported by the Special Committee of Regenerative Medicine. It is believed that mesenchymal stem cells will have a good effect in treating the sequelae of COVID-19 pneumonia through anti-inflammatory and tissue regeneration effects.”
It is reported that the COVID-19 pneumonia has spread on a large scale globally. With the increase of patients around the world, the risk has increased rapidly, and the third wave of pandemic has begun. Some patients with new coronavirus infection can cause severe sequelae even after treatment, such as brain fog, difficulty breathing and chest pain. According to an article published in the Journal of American Medical Association in 2020, 87.4% of COVID-19 patients were confirmed to have at least one sequelae effect, many of which were fatigue (53.1%), dyspnea (43.3%) and chest pain (21.7%) [1].

https://jamanetwork.com/journals/jama/fullarticle/2768351
Since patients with COVID-19 pneumonia were successfully treated, the World Health Organization has been deeply concerned about the long-term damage and long-term sequelae caused by COVID-19 pneumonia to the human body. Even after recovery, patients with COVID-19 pneumonia still have a continuous inflammatory response in their bodies. This has a great impact on the prognosis and recovery of patients with COVID-19 pneumonia.
Mesenchymal stem cells treat COVID-19 pneumonia and its sequelae
Mesenchymal stem cells have been shown to be effective in cell regeneration and reducing inflammatory factors in the body. Mesenchymal stem cells are adult stem cells with multidirectional differentiation potential. They can not only promote the regeneration and repair of damaged tissues, but also have good immune regulation capabilities. They can regulate inflammation by regulating the proliferation, differentiation and functional status of immune cells. Factor levels have good application prospects for various inflammation-related diseases [2]. Since the beginning of this year, domestic and foreign research results have been output in the field of stem cell treatment of COVID-19 pneumonia and its sequelae.

Picture from literature [3]
According to the literature [3], 7 patients with COVID-19 pneumonia (2 mild, 4 severe, and 1 critical) recruited by Beijing You’an Hospital in China received a single intravenous mesenchymal stem cell treatment. The results showed that within 14 days of transplantation , Mesenchymal stem cell therapy significantly improved the lung function of 7 patients, and there were no obvious adverse reactions. It is worth mentioning that within 2 days after mesenchymal stem cell treatment, all patients’ lung function and symptoms improved significantly. Within 2-4 days after treatment, all patients’ symptoms included: high fever, weakness, shortness of breath, and low blood oxygen saturation All disappeared, and the resting blood oxygen saturation was ≥95%. Two patients with mild illness and one patient with severe illness recovered and were discharged from the hospital within 10 days after treatment.
Internationally, recent data from the UAE Abu Dhabi Stem Cell Center (ADSCC) show that their stem cell therapy (UAECell19) has helped 5,000 patients recover from the new coronavirus infection. After the experiment, the researchers concluded that compared with patients receiving standard treatment, stem cell therapy (UAECell19) can shorten the hospital stay from 22 days to 6 days. Further analysis showed that patients receiving stem cell therapy were 3.1 times more likely to recover within 7 days than patients receiving standard treatment. Among patients receiving stem cell therapy, 67% of patients attributed their recovery to the new therapy.
In addition to mesenchymal stem cell transplantation, mesenchymal stem cell exosomes have also achieved good results in clinical research on the treatment of COVID-19 pneumonia. An article published in “Stem Cells and Development” pointed out that exosomes derived from mesenchymal stem cells have been effective in treating patients with severe COVID-19 pneumonia, with a recovery rate of 71%. Exosomes have the advantages of safety, restoration of oxygenation, down-regulation of cellular inflammatory factors, and reconstruction of immune system function. They are a promising drug candidate for the treatment of COVID-19 pneumonia [4].
Regarding the sequelae of COVID-19 pneumonia, in Japan, since August 1, 2020, the Japanese Respiratory Syndrome Society has been conducting research on the sequelae of COVID-19 pneumonia. In September, adipose tissue mesenchymal stem cells were collected from patients who had been treated for COVID-19 pneumonia but had sequelae. The patient receives 3 intravenous injections of 200 million stem cells every 2-4 weeks. The results of treatment found that the patient’s lung function, oxygenation index, inflammation level, pain degree and other symptoms were all relieved.
Outlook
Clinical research data shows that stem cell technology has become an effective way to tackle COVID-19 pneumonia. The Ministry of Science and Technology of the People’s Republic of China has repeatedly notified the clinical research and treatment effects of stem cell technology at the joint prevention and control press conference, and its safety and effectiveness have been confirmed, and many patients with severe COVID-19 pneumonia have been discharged from the hospital after treatment with stem cell technology. In addition to COVID-19 pneumonia, mesenchymal stem cells and their exosomes have also been applied to the treatment of cardiovascular diseases, nervous system diseases, immune system diseases and other diseases. Their safety and effectiveness have been continuously confirmed. I believe that in the near future In the future, stem cells will benefit more patients.
(sourceinternet, reference only)
In addition to mesenchymal stem cell transplantation, mesenchymal stem cell exosomes have also achieved good results in clinical research on the treatment of COVID-19 pneumonia. An article published in “Stem Cells and Development” pointed out that exosomes derived from mesenchymal stem cells have been effective in treating patients with severe COVID-19 pneumonia, with a recovery rate of 71%. Exosomes have the advantages of safety, restoration of oxygenation, down-regulation of cellular inflammatory factors, and reconstruction of immune system function. They are a promising drug candidate for the treatment of COVID-19 pneumonia [4].
Regarding the sequelae of COVID-19 pneumonia, in Japan, since August 1, 2020, the Japanese Respiratory Syndrome Society has been conducting research on the sequelae of COVID-19 pneumonia. In September, adipose tissue mesenchymal stem cells were collected from patients who had been treated for COVID-19 pneumonia but had sequelae. The patient receives 3 intravenous injections of 200 million stem cells every 2-4 weeks. The results of treatment found that the patient’s lung function, oxygenation index, inflammation level, pain degree and other symptoms were all relieved.
Outlook
Clinical research data shows that stem cell technology has become an effective way to tackle COVID-19 pneumonia. The Ministry of Science and Technology of the People’s Republic of China has repeatedly notified the clinical research and treatment effects of stem cell technology at the joint prevention and control press conference, and its safety and effectiveness have been confirmed, and many patients with severe COVID-19 pneumonia have been discharged from the hospital after treatment with stem cell technology. In addition to COVID-19 pneumonia, mesenchymal stem cells and their exosomes have also been applied to the treatment of cardiovascular diseases, nervous system diseases, immune system diseases and other diseases. Their safety and effectiveness have been continuously confirmed. I believe that in the near future In the future, stem cells will benefit more patients.
(sourceinternet, reference only)
Disclaimer of medicaltrend.org



