Clover Biology’s “S-trimer” COVID-19 candidate vaccine
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Clover Biology’s “S-trimer” COVID-19 candidate vaccine
Clover Biology’s “S-trimer” COVID-19 candidate vaccine. The next-generation COVID-19 vaccine? The Phase 1 clinical results of the clover biological protein subunit vaccine were released, and the global Phase II/III clinical trials are planned to be launched in the first half of 2021.
Clover Biology’s “S-trimer” COVID-19 candidate vaccine, when used in combination with adjuvants, showed good safety and tolerability in phase I clinical studies, and induced a strong neutralizing immune response.
Clover Biology plans to start global Phase II/III clinical trials in the first half of 2021, and is expected to release mid-term results of its vaccine effectiveness in mid-2021.
Shamrock Biopharmaceutical Co., Ltd. (hereinafter referred to as “Shamrock Bio”), a global clinical-stage biopharmaceutical company focusing on the research and development of innovative biological therapies and vaccines for the world’s serious diseases, today announced its “S-trimer” recombinant protein subunit COVID-19 When the candidate vaccine is used in combination with an adjuvant, the Phase I clinical study has shown good safety and immunogenicity. The positive result was published in the peer-reviewed journal “The Lancet” with the title “Next Generation COVID- 19 New coronavirus Vaccine: The Coming of Protein Vaccine” magazine special review, highly praised the “S-trimer” new coronavirus candidate vaccine.

The Phase I clinical study is a randomized, observer-blinded and placebo-controlled study in which 150 adult and elderly subjects participated in the test. The candidate vaccine was vaccinated in two doses, three weeks apart. The study aims to evaluate the “S-trimer” recombinant protein subunit COVID-19vaccine candidate in cooperation with Dynavax Technologies Corporation (hereinafter referred to as “Dynavax”, Nasdaq code: DVAX) CpG 1018 plus aluminum adjuvant or Ge The safety, reactogenicity and immunogenicity of LanxoSmithKline (hereinafter referred to as “GSK”, London Stock Exchange: GSK) vaccine adjuvant system to prevent pandemics when used in combination at multiple dose levels.
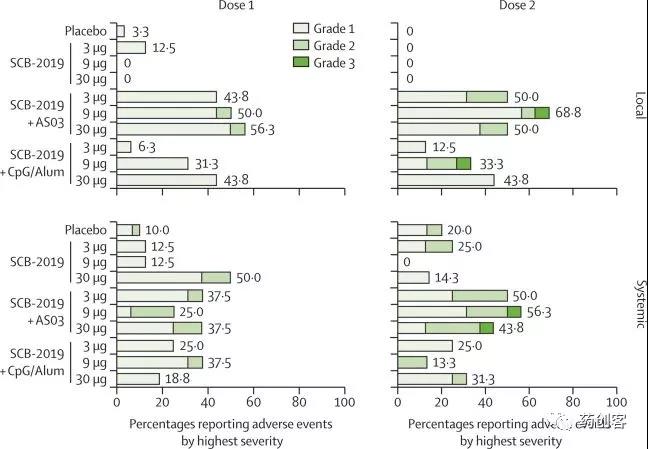
The research results show that the “S-trimer” COVID-19 candidate vaccine of Clover Biology is used in combination with two adjuvants, and both have good safety and tolerability, and there are no serious adverse reactions related to the research vaccine. At the same time, the two COVID-19 candidate vaccines both induced high levels of neutralizing antibodies that were equal to or higher than those observed in the serum of recovered patients, and had a clear partial Th1 cell immune response.
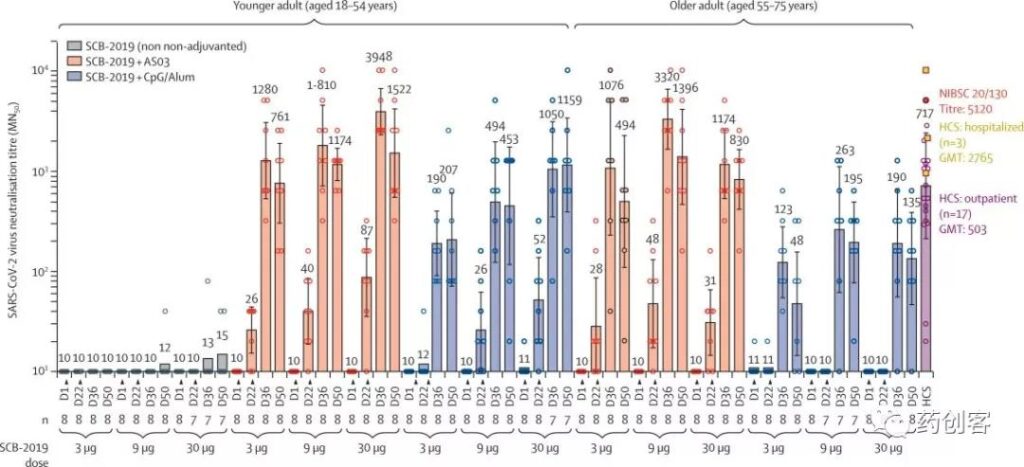
The “S-trimer” COVID-19 vaccine candidate and the combined adjuvant can be stable for a long time under the condition of refrigerator refrigeration temperature (2-8˚C), and stable at room temperature for at least two months, suitable for Distributed worldwide.

“The results of the Phase I dose exploratory study and adjuvant selection adaptability study show that the COVID-19 vaccine candidate used by the clover biological combined adjuvant has brought more value to the global COVID-19 vaccine product line. In adult and elderly subjects , Clover Biology’s COVID-19 vaccine candidate is safe, has a high neutralizing antibody titer, and has a good ratio of neutralizing/binding antibodies, which can trigger a cellular immune response. This makes us confident that Clover Biology’s COVID-19 vaccine candidate is suitable for development Further clinical research.”
Preclinical studies have shown that in the two animal challenge protection trials against the new coronavirus, the COVID-19 candidate vaccine of Clover Biology is protective. Based on the positive phase I clinical research results and large-scale production capacity, Clover Biological expects to carry out global phase II/III clinical trials in the next few months to further evaluate the “S-trimer” COVID-19 vaccine candidate and Dynavax The safety and efficacy of CpG 1018 plus aluminum adjuvant in combination.
The Epidemic Prevention Innovation Alliance (CEPI) will continue to fund the development of the clover biological “S-trimer” COVID-19 vaccine candidate until it is approved for marketing. Its total funding will be as high as 328 million US dollars, including the critical phase II/III clinical effect worldwide Sex test.
(source:internet, reference only)
Disclaimer of medicaltrend.org



