Central opioid receptor mediates morphine-induced pruritus and chronic pruritus
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Central opioid receptor mediates morphine-induced pruritus and chronic pruritus
Central opioid receptor mediates morphine-induced pruritus and chronic pruritus. Naloxone inhibits pruritus in both DNFB and CTCL models, while thiourea naloxone cannot, indicating the effect of central MORs on chronic pruritus.
ABSTRACT
Itching is a common side effect of opioids used in clinical treatment of pain. In this study, the authors demonstrated that intrathecal injection of the μ-opioid drugs morphine and DAMGO (a MOR selective agonist) can cause pruritus, which is mainly caused by spinal GABA inhibitory interneurons. MOR-mediated.
RESULTS
1. Intrathecal opioids induce acute itching through MOR on the surface of inhibitory interneurons.
Intrathecal injection of morphine induces pruritus in WT mice
Intrathecal injection of morphine caused a dose-dependent itching behavior in WT mice (Figure 1A, P = 0.0089). Morphine-induced pruritus mainly occurred within 30 minutes after injection. After pretreatment with the MOR selective antagonist CTOP, intrathecal morphine-induced pruritus was blocked, which indicated that the morphine-induced pruritus was mediated by MOR (Figure 1B, P = 0.0153).
Morphine-induced pruritus is mediated by inhibitory neurons in the spinal cord
Vgat-Cre or Sst-Cre mice were crossed with Oprm1fl/fl mice to knock out MOR on inhibitory or excitatory interneurons in the spinal cord, respectively. It was found that the knockout of Oprm1 on inhibitory neurons completely eliminated morphine-induced pruritus (Fig. 1C. P = 0.0094), while the knockout of Oprm1 on excitatory interneurons did not affect morphine-induced pruritus. (Figure 1D. P = 0.4476). It shows that intrathecal injection of morphine acts on the MOR on the inhibitory interneurons, resulting in an itching response.
DAMGO can induce scratching behavior
The scratching behavior induced by intrathecal DAMGO showed a bell-shaped dose response curve, and itching mainly occurred in the first 5 minutes (Figure 1E). Like morphine, DAMGO-induced pruritus was significantly eliminated in mice that knocked out inhibitory interneurons MOR. (Figure 1F, P=0.0075).
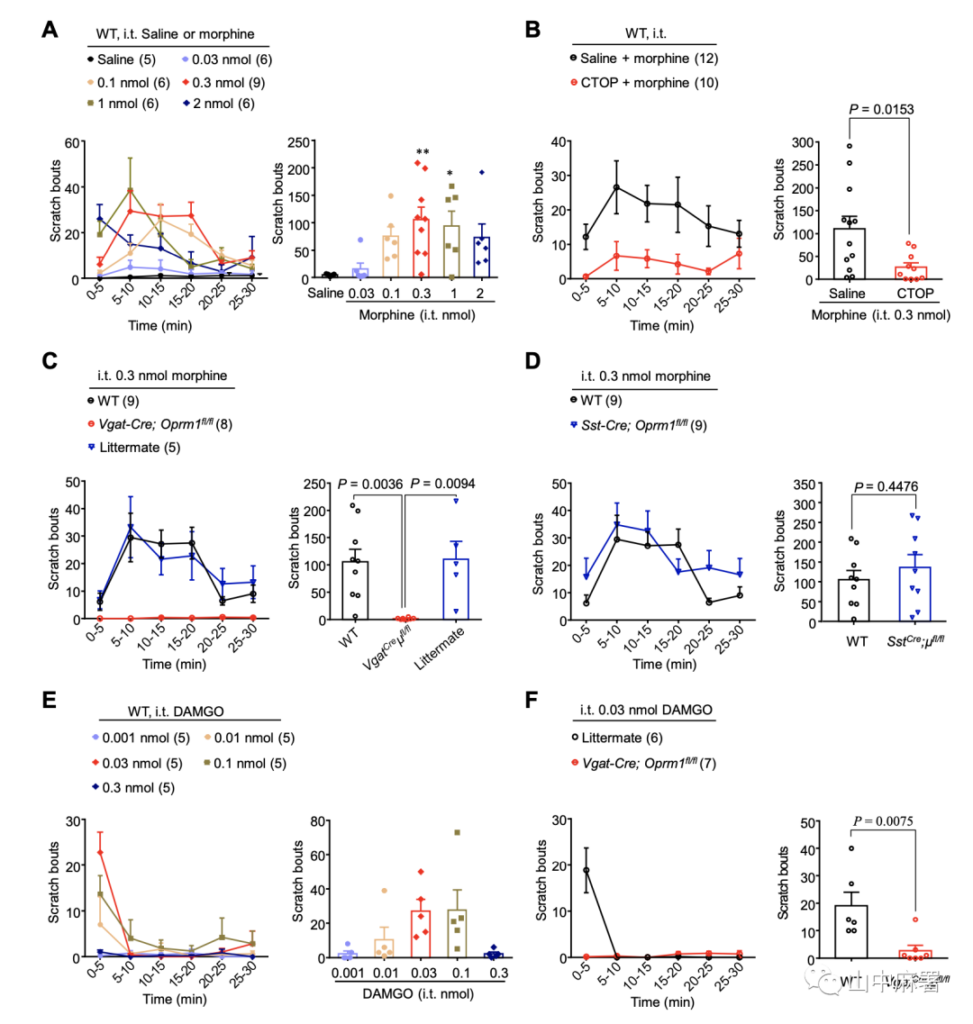
▲ Figure 1
2. The respective roles of peripheral and central MOR in the process of morphine-induced pruritus
The researchers used an opioid receptor antagonist that does not pass through the blood-brain barrier: thiourea naloxone. Pre-injection of thiourea naloxone through the intraperitoneal cavity only blocked the MOR activity of peripheral sensory neurons and did not change the morphine induction Itching (Figure 2A, P = 0.1873). In contrast, intrathecal thiourea naloxone completely eliminated morphine-induced pruritus, indicating that MOR signals in the spinal cord mediate morphine-induced pruritus (Figure 2B, P = 0.0004). Furthermore, in mice with MOR knockout on TRPV1+ sensory neurons and mice pretreated with resininferatoxin (RTX, ablation of TRPV1+ sensory neurons), morphine-induced pruritus did not change. (Figure 2C, D, P = 0.2005, P = 0.4951). In addition, intradermal injection of morphine (100nmol) can induce a very mild itching response (an average of 14 scratches within 30 minutes), and this response was unchanged in Vgat-Cre; Oprm1fl/fl mice (Figure 2E, P = 0.3281). In summary, the pruritus induced by intrathecal injection of morphine is specifically mediated by inhibitory interneurons MOR in the spinal cord.
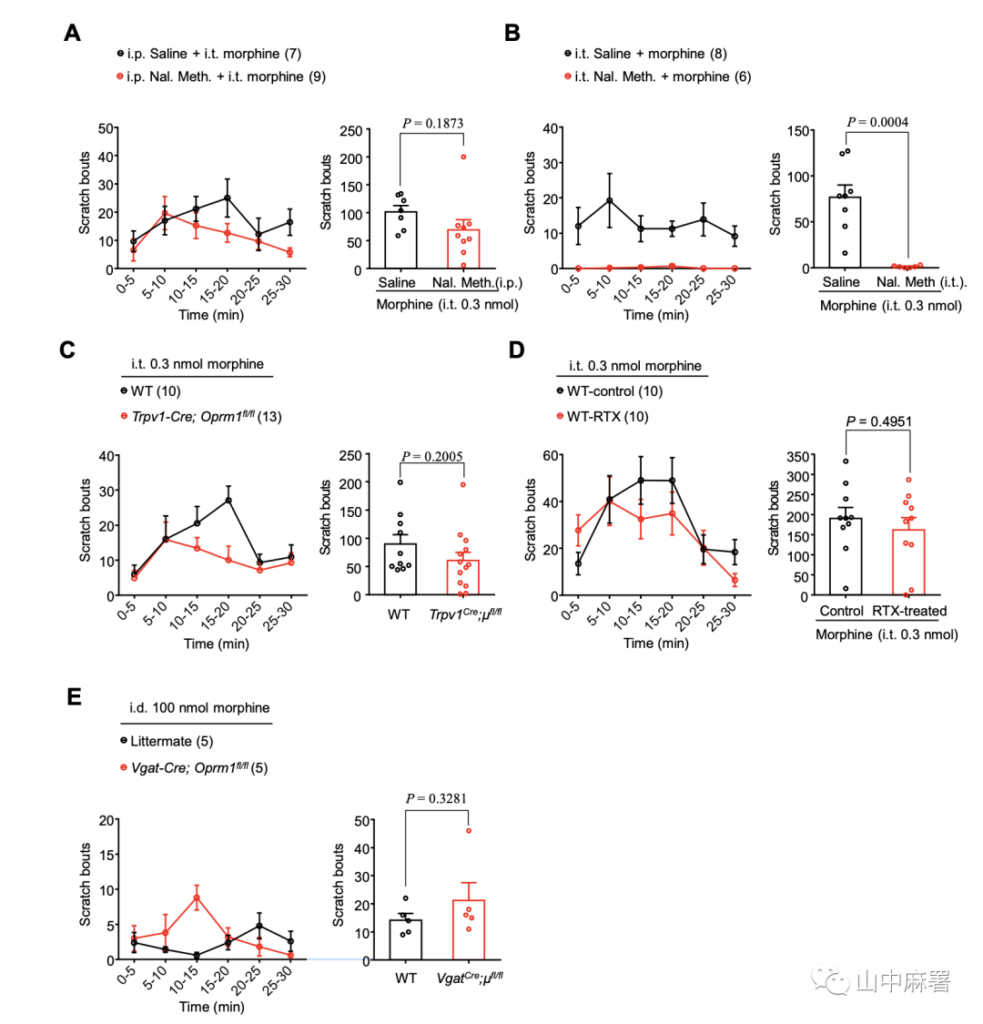
▲ Figure 2
3. Intrathecal morphine induces peripheral sensory neurons and spinal cord excitatory interneurons to produce anti-pain effects through MOR.
The intrathecal administration of opioids is widely used clinically for analgesia. Conditional knockout of MOR in TRPV1+, Vgat+, and SST+ neurons did not change the baseline value of tail flick time (Supplementary Figure 1A, P> 0.9999, P = 0.1324, P = 0.2199), nor did it change the hot plate shrinkage time (Supplementary Figure 1B, P = 0.7716, P> 0.9999, P> 0.9999). In the tail flick test, intrathecal injection of morphine significantly increased the tail flick time. This analgesic effect was significantly reduced in Sst-Cre; Oprm1fl/fl mice (Figure 3A, P = 0.0016), but in Vgat- Cre; Oprm1fl/fl (Figure 3A, P = 0.4893) or Trpv1-Cre; Oprm1fl/fl mice (Figure 3A, P = 0.0801) did not decrease. In the hot plate test, in SstCre; Oprm1fl/fl and Trpv1-Cre; Oprm1fl/fl mice, the analgesic effect induced by intrathecal morphine was significantly reduced (Supplementary Figure 1C, both P<0.0001). In Vgat-cre; Oprm1fl/fl mice, intrathecal morphine-induced antipain was significantly enhanced (Supplementary Figure 1C, P=0.050). In summary, the analgesic effect induced by intrathecal morphine is mediated by the MOR of spinal cord excitatory interneurons and peripheral sensory neurons.
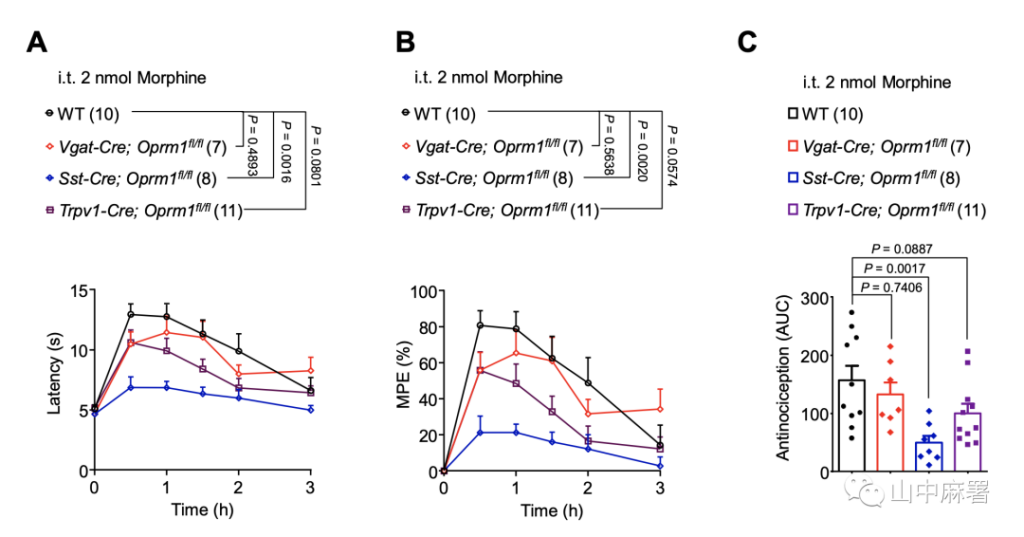
▲ Figure 3
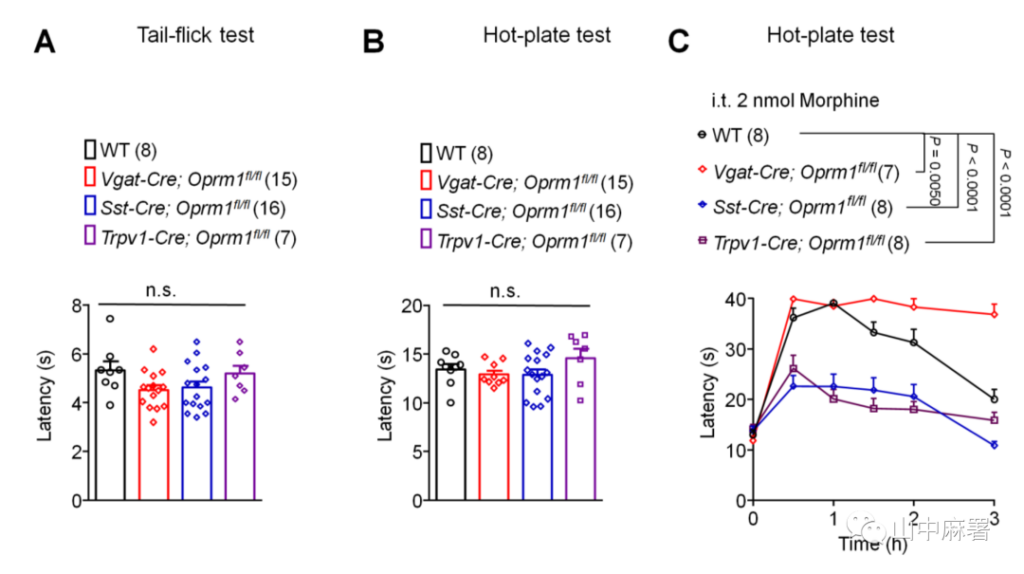
▲ Figure S1
4. MOR agonists inhibit the activity of MOR+ inhibitory interneurons in spinal cord tablets.
Expression of MOR in the spinal dorsal horn (SDH)
The expression of MOR in the spinal dorsal horn (SDH) was detected by RNAscope. In Vgat-Cre; Ai9 tdTomato mice, the expression of Oprm1 on excitatory and inhibitory neurons was detected by staining with Vglut2 mRNA (SLC17a6). The expression of Oprm1 was observed on both Vgat+ and Vglut2+ interneurons in layer II of SDH (Figure 4A-C). This finding is consistent with published single-cell sequencing data, that MOR is widely expressed in the inhibitory and excitatory interneurons of SDH. However, the expression level of MOR in inhibitory interneurons was higher than that of excitatory interneurons (Supplementary Figure 5A).

▲ Figure 4

▲ Figure S5
Influence of MOR agonists on inhibitory interneuron activity
Previous studies have shown that opioids activate potassium channels through G protein-coupled receptors and generate outward currents in neurons expressing MOR. This study shows that DAMGO (0.5μM) induces outward currents in 9 neurons in 19 SDH layer II inhibitory interneurons, in Vgat-Cre; Ai9 tdTomato mouse spinal cord slices of Vgat+ interneurons , The amplitude is 19.5pA (Figure 5A). Morphine perfusion (10μM) significantly inhibited the evoked action potentials of SDH layer II inhibitory interneurons (Figure 5B and C, P = 0.0109).
Cross the Vgat-Cre mice with Ai32 mice to express the channel rhodopsin ChR2 in the spinal cord Vgat+ interneurons. When the spinal cord Vgat+ interneurons are stimulated by blue light with a wavelength of 473 nm, the inhibitory postsynaptic currents (IPSCs) induced in the second layer Vgat+ interneurons are recorded. Morphine (10 μM) can significantly inhibit the induced IPSCs, indicating that the function of inhibitory interneurons is inhibited (Figure 5D and E, P<0.0001). In summary, μ-opioids can directly inhibit the activity of MOR+ inhibitory interneurons, indicating that opioid-induced pruritus is the result of inhibiting MOR+ inhibitory interneurons.

▲ Figure 5
5. Intrathecal morphine-induced pruritus was suppressed by NPY, but was abolished by GRPR+interneuron ablation.
Two of SDH inhibitory interneurons, namely NPY+ and dynorphin (Dyn)+interneurons, are thought to be involved in the gate control of itch. The author first detected the expression of Oprm1, Npy and Pdyn mRNA in SDH by triple in situ hybridization, thereby detecting the expression of MOR in these two inhibitory interneuron populations. The authors found that MOR in inhibitory interneurons is mainly expressed in NPY+ interneurons (Supplementary Figures 2A and B). The co-expression level of MOR and NPY is similar to the co-expression level of MOR and Vgat, which indicates that MOR in inhibitory interneurons is mainly co-expressed with NPY. This is consistent with the data from the previous single-cell sequencing database (Supplementary Figure 5). It is worth noting that the combined administration of NPY (10 µg) and morphine (0.3 nmol) in the sheath can significantly inhibit morphine-induced itching (Figure 6A, P <0.0001).
NPY-Y1 receptor is involved in the transmission of itching
Y1R and GRP are highly co-expressed (70%) (Supplementary Figure 3). The expression patterns of MOR, NPY and Y1 indicate that the activation of MOR in NPY+ inhibitory interneurons may inhibit GRP+ interneurons and cause itching. To test this hypothesis, bombesin-saporin (400 ng) was injected intraperitoneally to ablate GRPR+ interneurons in SDH. In the mice pretreated with bombesin-saporin, the itching induced by sheath injection of GRP (0.1 nmol) was eliminated, indicating the successful ablation of GRPR+ interneurons (Figure 6B, P = 0.0049). It is worth noting that ablation of GRPR+ neurons can completely eliminate intrathecal morphine-induced pruritus, indicating that GRPR+ interneurons play an important role in morphine-induced pruritus (Figure 6C, P = 0.0072). In contrast, in Vgat-Cre; Oprm1fl/fl mice, intrathecal GRP-induced itch was not affected (Figure 6D, P=0.7797). RNAscope data showed that the co-expression level of MOR with GRP+ interneurons (29.4%) and GRPR+ interneurons (4.7%) was low (Supplementary Figure 4), which is consistent with single-cell sequencing data (Supplementary Figure 5). In summary, in intrathecal morphine-induced pruritus, the role of GRP/GRPR is located downstream of MOR+ inhibitory interneurons.

▲ Figure 6

▲ Figure S2

▲ Figure S4
6. MOR in inhibitory interneurons contributes to the persistent itching associated with dermatitis, but does not contribute to the occurrence of acute chemical itching.
Contribution of MOR to pruritus in different animal models of pruritus
A DNFB-induced model of allergic contact dermatitis was used to determine the role of MOR in chronic pruritus. In Vgat-Cre; Oprm1fl/fl mice, DNFB-induced pruritus was significantly reduced (Figure 7A and B, P = 0.0224). In addition, the authors also tested acute itching induced by chloroquine (CQ) and histamine. Acute pruritus induced by intradermal injection of CQ and histamine did not change significantly in Vgat-Cre; Oprm1fl/fl mice (Figure 7C and D, P=0.6328, P=0.5120). These results indicate that MOR on inhibitory interneurons specifically contributes to chronic pruritus associated with dermatitis, but does not have a positive effect in acute chemical pruritus.

▲ Figure 7
Central but not peripherally restricted MOR antagonists inhibit chronic pruritus induced by dermatitis and lymphoma
Intraperitoneal injection of naloxone can significantly reduce spontaneous pruritus in the DNFB model, while thiourea naloxone cannot, which suggests that MOR expressed in the central nervous system is more critically involved in chronic pruritus (Figure 8A, P = 0.0044, respectively) And P = 0.6383). In addition, intrathecal injection of naloxone significantly reduced DNFB-induced pruritus (Figure 8B, P = 0.0007).
Mouse skin T-cell lymphoma (CTCL) model. In the CTCL model, mice develop lymphoma and chronic itching. Similar to the results found in the DNFB model, only systemic treatment with naloxone, rather than thiourea naloxone, can significantly inhibit spontaneous pruritus in CTCL mice (Figure 8C, P <0.0001 and P = 0.2987, respectively ). Intraperitoneal injection of naloxone can also significantly abolish spontaneous pruritus in the CTCL model (Figure 8D, P = 0.0001). These results indicate that, compared with peripheral MOR, central MOR plays an important role in chronic pruritus induced by dermatitis and lymphoma.

▲ Figure 8
CONCLUSION
The author’s findings indicate that intrathecal opioids act on the MOR of inhibitory interneurons in the spinal cord to regulate itching by releasing inhibition.
μ-opioids can inhibit the activity of Vgat+ interneurons in the spinal cord, relieve its inhibition of the itching signal pathway, and cause itching.
After the deletion of Oprm1-Vgat, chronic pruritus in a mouse model of DNFB-induced allergic contact dermatitis was reduced.
Naloxone inhibits pruritus in both DNFB and CTCL models, while thiourea naloxone cannot, indicating the effect of central MORs on chronic pruritus.
Central opioid receptor mediates morphine-induced pruritus and chronic pruritus
Central opioid receptor mediates morphine-induced pruritus and chronic pruritus
(source:internet, reference only)
Disclaimer of medicaltrend.org



