Padcev: Significantly improve the survival of patients with bladder cancer
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Padcev: Significantly improve the survival of patients with bladder cancer
Padcev: Significantly improve the survival of patients with bladder cancer. Phase 3 clinical results of “first-in-class” antibody conjugate drugs.
A few days ago, Seagen (formerly Seattle Genetics) and Astellas Pharma jointly announced the results of a Phase 3 clinical trial of an innovative antibody-conjugated drug Padcev (enfortumab vedotin-ejfv) for the treatment of locally advanced or metastatic urothelial cancer patients Published in the New England Journal of Medicine (NEJM).
The scheduled interim analysis showed that among patients who received chemotherapy and PD-1/PD-L1 inhibitor treatment, the median overall survival of patients who received Padcev was 12.9 months, which was 3.9 months longer than that of the chemotherapy control group (HR =0.70, 95% CI: 0.56-0.89, p=0.001).
Based on this result, the two companies will submit a regulatory application to the FDA, seeking to convert accelerated approval to full approval. At the same time, this evidence will support Padcev’s global regulatory application.

Globally, in 2020, approximately 580,000 people worldwide will be diagnosed with bladder cancer. Urothelial cancer accounts for 90% of all bladder cancers and can also occur in the renal pelvis, ureter, and urethra. After the failure of initial platinum-containing therapy, 80% of advanced patients will not respond to PD-1 or PD-L1 inhibitor therapy.
Padcev is an antibody conjugate drug that conjugates anti-nectin-4 monoclonal antibody and microtubule inhibitor. Nectin-4 protein is a cell adhesion molecule highly expressed in urothelial carcinoma. Based on the response rate data, this therapy was approved by the US FDA in December 2019 to treat patients with locally advanced or metastatic urothelial cancer. These patients have been treated with platinum-containing chemotherapy and PD-1/PD-L1 inhibitors.
Trial data published in NEJM showed that when the median follow-up time reached 11 months, Padcev reduced the risk of death by 30% compared with chemotherapy.
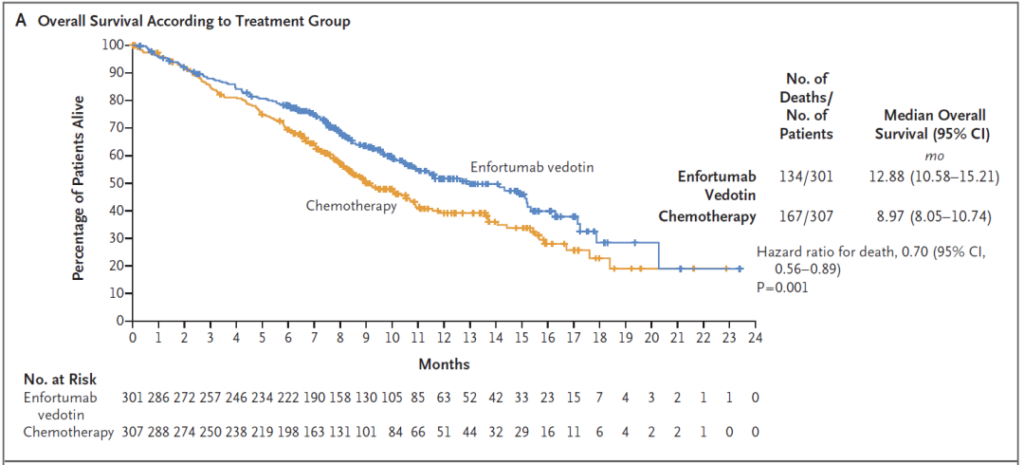
▲Padcev’s overall survival data in phase 3 clinical trials (picture source: reference [2])
And Padcev also reached multiple secondary endpoints of this clinical trial. For example, Padcev reduced the patient’s risk of disease progression or death by 38%. The median progression-free survival in the Padcev group was 5.55 months and that of the chemotherapy group was 3.71 months. The overall confirmed response rate in the Padcev group was 40.6%, and the disease control rate reached 71.9%.
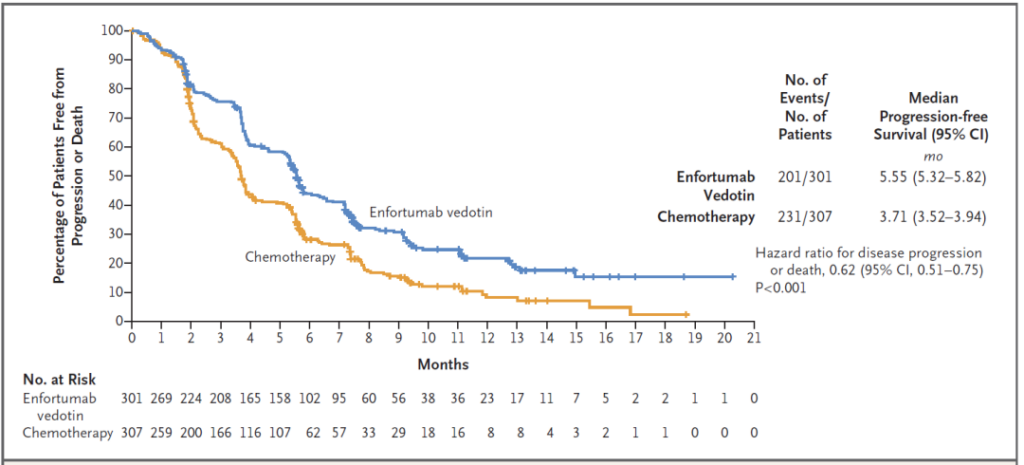
▲Padcev’s progression-free survival data in phase 3 clinical trials (picture source: reference [2])
Dr. Roger Dansey, Chief Medical Officer of Seagen, said: “Since the accelerated approval of the FDA at the end of 2019, Padcev has been incorporated into medical practice by doctors. These confirmatory results further prove the benefits of this therapy for patients with advanced bladder cancer.
(source:internet, reference only)
Disclaimer of medicaltrend.org



