The plasma of recovered patients cannot improve COVID-19 survival rate
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The plasma of recovered patients cannot improve COVID-19 survival rate
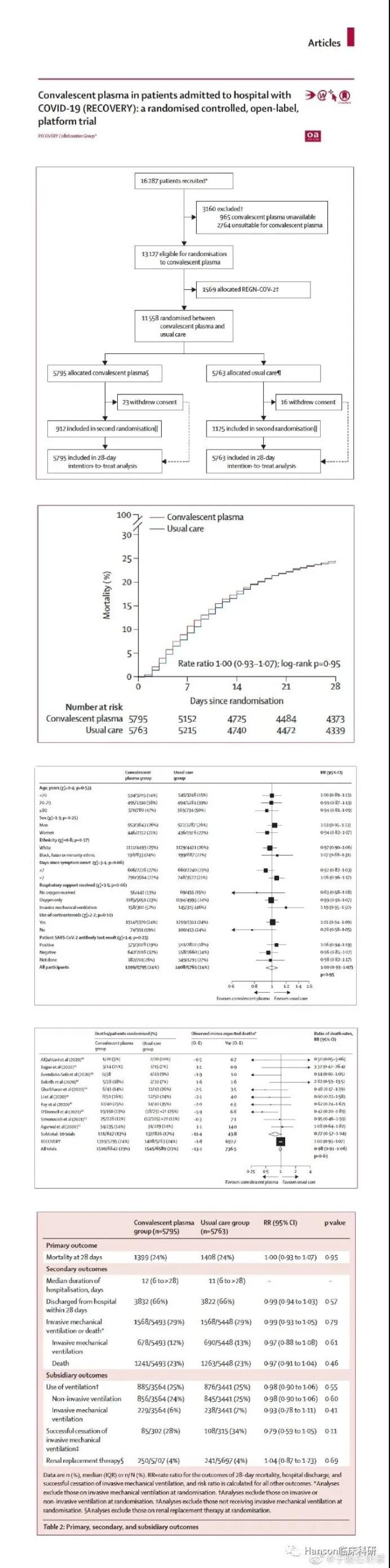
The plasma of recovered patients cannot improve COVID-19 survival rate. On May 14, 2021, the well-known British RECOVERY research group published the largest clinical research results of the recovered plasma in Lancet, which is also one of the largest RCT clinical trials for the treatment of COVID-19 to date.
This research is of decisive guiding significance for the future development of plasma of recovered patients.
This study shows that the plasma of recovered patients is not beneficial to the treatment of COVID-19 in hospital, but on the other hand, it can also show that the polyclonal SARS-CoV-2 plasma product has no ADE effect.
This RECOVERY study enrolled 16,287 patients from May 28, 2020 to January 15, 2021; 11,558 patients were randomly assigned, 5,795 received plasma from recovered patients, and 5763 received standard treatment.
The study found that there was no difference in 28-day mortality between the two groups. 1399 patients in the plasma group (24%) of the recovered patients and 1408 patients (24%) in the standard treatment group died within 28 days (RR = 1, p=0.95). 3,832 (66%) people in the plasma treatment group of recovered patients were discharged from the hospital, and 3,822 (66%) people in the standard treatment group were discharged from the hospital (RR = 0.99, p=0.57). 1568 (29%) people in the plasma treatment group of the recovered patients received mechanical ventilation and died, and 1568 (29%) people in the standard treatment group received mechanical ventilation and died (RR = 0.99, p=0.79).
Studies have found that among hospitalized COVID-19 patients, high-titer recovered plasma cannot improve the survival rate of hospitalized COVID-19 or other predetermined clinical outcomes. But on the other hand, it can also indicate that the polyclonal SARS-CoV-2 plasma product has no ADE effect. From the results of such a large-scale clinical trial, it can be predicted that SARS-CoV-2 may not have an ADE effect.
Editor’s note:
The RECOVERY study is the most important clinical trial during the COVID-19 epidemic, and there is no one.
It was confirmed for the first time that dexamethasone was effective in treating critically ill COVID-19, and the noise of hydroxychloroquine in the treatment of COVID-19 was ruled out.

(Results of RECOVERY research)
This trial is also of great significance. It not only confirms that among hospitalized COVID-19 patients, high-titer recovered plasma cannot improve the survival rate of hospitalized COVID-19 or other predetermined clinical outcomes, thereby avoiding more waste; at the same time, it can also explain The polyclonal SARS-CoV-2 plasma product has no ADE effect. From the results of such a large-scale clinical trial, it can be predicted that SARS-CoV-2 may not have an ADE effect.
More importantly, from the RECOVERY study, we can learn the core of high-quality clinical trial design: multi-center, large sample, randomized double-blind placebo-controlled.
In the face of a sudden COVID-19 epidemic, it is most important to set the end-point event as a simple and easy-to-obtain indicator, such as the case fatality rate in this study.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



