General design of beta-coronavirus vaccines: COVID-19 MERS and SARS
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
General design of beta-coronavirus vaccines: COVID-19 MERS and SARS
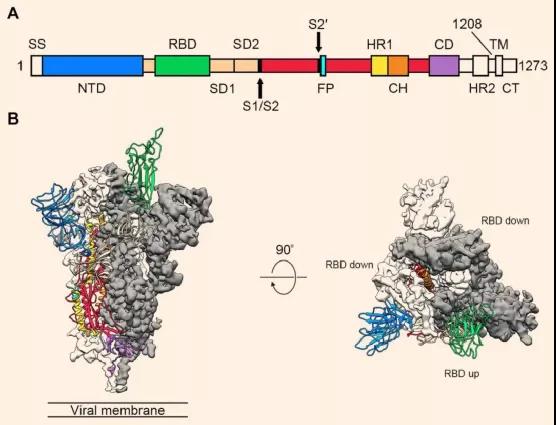
General design of beta-coronavirus vaccines: COVID-19 MERS and SARS. The spike (S) protein on the surface of the coronavirus is in a trimeric conformation and is responsible for binding to host cell receptors and mediating virus invasion.
Therefore, the S protein is the main protective immunogen of the coronavirus. There is a receptor binding region (RBD) on the S protein that is directly responsible for docking with the host receptor, which is a very attractive vaccine target for coronavirus. It stimulates the production of neutralizing antibodies, blocks the binding of the virus to the receptor, realizes immune focusing, and is expected to avoid the potential antibody dependence enhancement risk of coronavirus immunity.
However, due to the small molecular weight of RBD and limited immunogenicity, the vaccine designed with it has a weak ability to induce the body to produce neutralizing antibodies. However, most potent neutralizing monoclonal antibodies currently target CoV RBD. Therefore, RBD is an attractive vaccine target because it can focus the immune response on the interference of receptor binding. So far, there have been reports that some RBD-based vaccines are being developed to combat MERS-CoV and SARS-CoV.
So far, there are 6 types of coronaviruses that can infect humans. The new coronavirus that ravaged the world last year is the seventh coronavirus that is known to infect humans. The remaining six are HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, SARS-CoV (causing severe acute respiratory syndrome) Sign) and MERS-CoV (causing Middle East Respiratory Syndrome).
It is understood that about 30% of upper respiratory tract infections worldwide are caused by the four coronaviruses HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1. These four coronaviruses are second only to rhinoviruses that cause the common cold. Two causes. The other three are highly pathogenic coronaviruses, all of which cause severe respiratory diseases in humans, and all belong to β-coronaviruses.
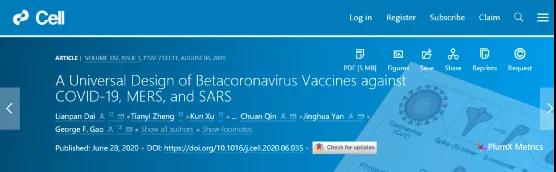
In June last year, the top international academic journal “Cell” published online a study completed by Gao Fu, director of the Chinese Center for Disease Control, academician of the Institute of Microbiology, Chinese Academy of Sciences, and others, entitled “A universal design of betacoronavirus vaccines against COVID-19, MERS and SARS” provides a universal design for β-coronavirus vaccines against COVID-19, MERS and SARS.
Basically two ways of producing vaccines
There are basically two ways of producing vaccines. One is gene-based vaccines, such as new coronavirus DNA or mRNA vaccines, and recombinant vector vaccines. These vaccines can use host cells to replicate virus-related genes and generate antigens after entering the human body. Make people immune.
The other is a more classic vaccine production method, which uses viral proteins or protein subunits to make vaccines. These proteins are directly mass-produced in vitro and injected into the human body to provide immunity. The hepatitis B vaccine is prepared in this way. This traditional method of activating immunity with protein is relatively more effective and safer.
Among the coronaviruses, the most suitable vaccine to target is the S protein, which is often referred to as the spike protein. Conventional vaccine production methods can directly use S protein as the antigen component of the vaccine. The complete S protein is very large (about 600 kDA) and usually has high immunogenicity, that is, it can make the human body produce a strong immune response.
A few years ago, researchers at the Scripps Research Institute in the United States used the S protein of the MERS virus to design a vaccine against MERS. In their experiments, this vaccine can stimulate the production of a large number of neutralizing antibodies. This shows that the traditional vaccine preparation method is sufficient to deal with the invasion of coronavirus.
However, vaccines produced with S protein have a drawback. S protein includes RBD (receptor binding domain) and non-RBD, such as the N-terminal domain. These regions can enable the body to produce neutralizing antibodies, while other protein regions It is possible to produce non-neutralizing antibodies, leading to ADE effects. ADE is the so-called antibody-dependent enhancement. In this case, the antibodies (mostly non-neutralizing antibodies) produced can not only eliminate the virus, but also enhance the virus’s ability to invade cells. This is a phenomenon that has been observed in SARS and MERS patients.
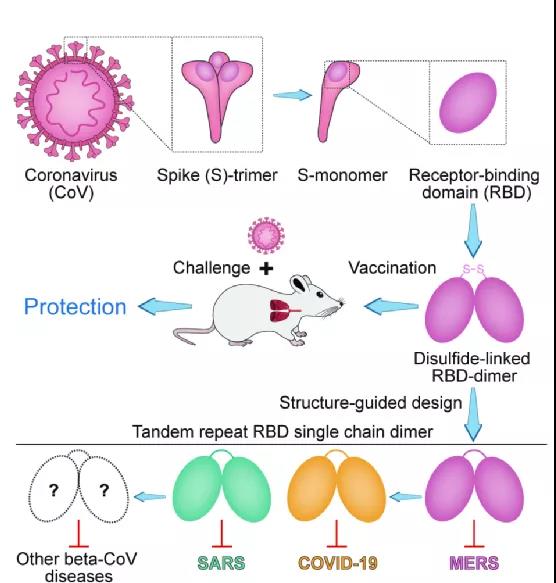
(▲Design idea: split the S protein trimer to obtain RBD, and use disulfide bond to form RBD dimer. Using this as a vaccine component, when the vaccinated mice receive coronavirus infection, the immune system can effectively prevent the virus Invade, protect the individual.)
Strategy
The Gaofu team started the research and development of the MERS coronavirus vaccine several years ago. In this study, in order to overcome this immunogenicity limitation, the research team constructed the MERS coronavirus RBD dimer antigen. They found that this RBD dimer connected by disulfide bonds can induce higher neutralizing antibodies than the traditional monomeric form, and protect mice against MERS coronavirus infection and relieve lung pathological damage.
Immediately afterwards, X-ray crystal structure analysis revealed that the MERS coronavirus RBD dimer completely exposed the dual receptor binding motif, which is also the main target of neutralizing antibodies. In order to obtain a more stable and uniform dimer antigen, the research team further optimized the dimer protein design based on the structure, and obtained a tandem repeat RBD single-chain dimer (RBD-sc-dimer).
The expression form of this tandem repeat single-chain dimer is uniform and does not contain foreign sequences. Compared with the previous version, the efficacy of the disulfide-linked RBD dimer vaccine is maintained or even improved.
The universal β-CoV immunogen design of Gao Fu and other researchers overcomes the immunogenicity limitations of RBD-based vaccines. People have previously observed CoV RBD dimers, but their immunogenicity has not been tested. They found that compared with the traditional RBD monomer form, the RBD dimer form linked by disulfide bonds significantly enhanced the antibody response and neutralizing antibody titers. In mouse models, it protects these animals from MERS-CoV infection and relieves lung damage.
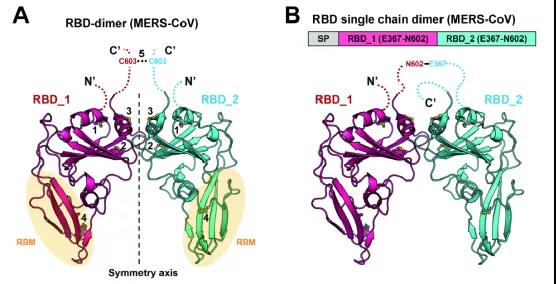
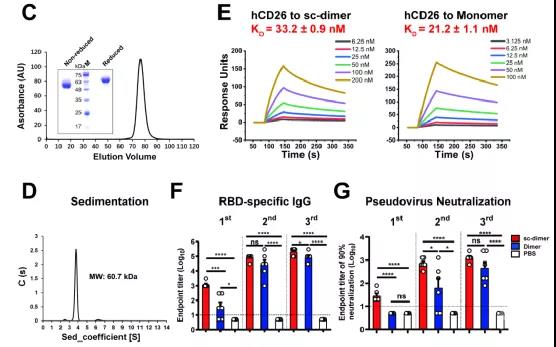
The research first used the MERS coronavirus as the test target, and the RBD of MERS-CoV was expressed in large quantities in insect cells. These collected RBD monomers, in order to improve the stability of this dimer, without introducing any foreign sequence, through structure-guided design, this immunogen is further designed as a tandem repeat single-chain dimer (Tandem repeat single chain dimer, tandem repeat sc-dimer) version. After the disulfide bond treatment, the RBD dimer is formed.After purification and concentration adjustment, the RBD dimer becomes the final vaccine component.
Compared with mice injected with RBD monomer vaccine, mice injected with RBD dimer vaccine will have stronger immunity and higher titers of neutralizing antibodies in the body. Those mice that are themselves susceptible to the MERS virus can sustainably resist MERS virus infection after receiving this new vaccine injection.
Next step
The research team also extended this strategy to the design of vaccines against COVID-19 and SARS, increasing the NAb titer by 10-100 times. RBD-sc-dimer has obtained high yield in pilot scale production, which provides support for further clinical development.
Gao Fu and others pointed out in the paper that the immunogen design framework can be universally applied to other β-coronavirus vaccines to deal with new threats that may arise in the future.
In particular, the neutralizing antibody titer against SARS-CoV-2 infection triggered by two doses of RBD-sc-dimer was as high as about 4096. The RBD-sc-dimer of MERS-CoV and SARS-CoV-2 has been further developed for pilot-scale production in GMP-level production.
This result has given a lot of inspiration to researchers such as Gao Fu, who believe that this method can also deal with SARS and the new coronavirus. In follow-up research, they designed an upgraded version of RBD dimer, using multiple dimers to form a tandem form of RBD dimer (sc-dimer). The sc-dimer has the same immune function as a single dimer. But the immunization efficiency has been improved again.
In addition to MERS coronavirus, this improved sc-dimer is also suitable for SARS coronavirus and new coronavirus. The neutralizing antibody titer produced by the vaccine produced by sc-dimer in mice is 10-100 times that of the traditional monomeric RBD vaccine. This means that this RBD dimer tandem method is suitable for various β-coronaviruses and can be said to be a universal design method for the production of SARS-CoV, MERS-CoV and SARS-CoV-2 vaccines.
Breakthrough
Based on this breakthrough in basic research, the Institute of Microbiology of the Chinese Academy of Sciences immediately reached a cooperation intention with a partner company to jointly develop a new coronavirus recombinant subunit protein vaccine, and has independent intellectual property rights for the vaccine. The vaccine is one of the five technical routes deployed by the State Council in response to the joint prevention and control mechanism of the new coronavirus pneumonia epidemic.
The vaccine will complete Phase I and Phase II clinical trials in October 2020. The results showed that after the whole course of vaccination of the vaccine, no serious adverse reactions occurred, which conformed to the characteristics of small adverse reactions of subunit vaccines, and the level of neutralizing antibodies produced was equivalent to the current international recombinant protein vaccines and mRNA COVID-19 vaccines, reaching the international advanced level.
Starting from November 2020, the vaccine has successively launched phase III clinical trials in China and in Uzbekistan, Pakistan, Ecuador, and Indonesia, with 29,000 patients planned to be vaccinated. According to the Institute of Microbiology, Chinese Academy of Sciences, the current phase III clinical trials are progressing smoothly.
It is worth noting that, according to the introduction of the Institute of Microbiology of the Chinese Academy of Sciences, the vaccine production uses engineered cells (CHO) to produce recombinant proteins, does not require high-level biosafety laboratory production workshops, and the production process is stable and reliable, and can quickly achieve large-scale domestic and foreign Industrialized production has significantly reduced the cost of vaccine production, and storage and transportation are convenient.
The first recombinant subunit protein vaccine in the world
The Institute of Microbiology, Chinese Academy of Sciences announced on March 15, 2021 that, on March 10, the recombinant novel coronavirus vaccine (CHO cell) jointly developed by the Institute of Microbiology of the Chinese Academy of Sciences and a partner company was approved for emergency use in China and became a domestic The fourth new coronavirus vaccine approved for emergency use.
This is also the first new coronavirus recombinant subunit protein vaccine approved for clinical use in the world.

(source:internet, reference only)
Disclaimer of medicaltrend.org



