Prospects and development of DNA vaccines against new coronavirus
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Prospects and development of DNA vaccines against new coronavirus
Prospects and development of DNA vaccines against new coronavirus. Compared with inactivated virus attenuated vaccines and genetically recombined virus protein vaccines, DNA vaccines are characterized by the ability to establish a manufacturing process within a short period of time. However, so far, although no DNA virus vaccine has officially entered clinical application in the world.
Research teams from Brazil and Germany published an article titled “DNA vaccines against COVID-19: Perspectives and challenges” in the journal “Life Sciences”.
The author pointed out that DNA vaccines are composed of genes or fragments encoding immunogenic antigens delivered to host cells by using DNA plasmids as carriers. This method can effectively induce humoral and cell-mediated immune responses. The formulation of the vaccine should enable the transfer of genetic material to the nucleus of the host.
Coronavirus is a single-stranded positive-stranded RNA virus without reverse transcriptase. The virus belongs to the coronavirus family (Nidovirales). The Coronavirus family contains four genera, including alpha-coronavirus (alphaCoV), beta-coronavirus (betaCoV), delta-coronavirus (deltaCoV) and gamma-coronavirus (gammaCoV). Structurally speaking, SARS-CoV-2 contains four structural proteins, including spikes (S), envelope (E), membrane (M) and nucleocapsid (N). In addition, these proteins have a high degree of sequence similarity with SARS-CoV and MERS-CoV proteins.
Therefore, the knowledge of SARS-CoV and MERS-CoV vaccines provides some assistance for the development of SARS-CoV-2 vaccine design, thereby helping to accelerate the development of new vaccines for COVID-19.
At this stage, some DNA vaccines expressing S, M and N proteins against SARS-CoV have been successfully developed. Related results confirmed the strong protective humoral and cellular immune responses in mice, macaques and camels. In addition, GLS-5300, the first anti-MERS-CoV DNA vaccine candidate to enter clinical trials, is well tolerated and has no serious adverse events related to the production of the vaccine. The immune response has nothing to do with dose, was detected in more than 85% of participants after two immunization regimens, and can last for one year.
Clinical trials for DNA vaccines
In the genome of the new coronavirus released in January last year, the author believes that during the infection process, antibodies against the N and S proteins are mainly produced. The N protein covers the viral genome and also participates in the release of viral particles from the cell. The S protein binds to the host cell through its receptor binding domain, and thus begins to infect the host cell, thereby playing an important role in the pathogenesis. The S protein has 1273 amino acid residues and can be divided into three subunits S1, S2 and S2′. Each subunit is different in the process of adhesion to host cells.
The S1 subunit interacts with human ACE2 and participates in the attachment of the virion to the host cell membrane, thereby initiating the infection process. In addition, during this process, the S protein undergoes conformational changes caused by its entry into the endosome of the host cell. The S2 subunit acts as a fusion protein and helps the virus and the host cell membrane to bind. During the fusion process, the S2 protein appears in three consecutive conformational states: 1) pre-fusion (local state) 2) pre-hairpin (over state) 3) subsequent fusion (hairpin state). Subsequently, the surface protease cleaves S2. The SARS-CoV-2 S protein has a furin site as an additional cleavage site, which may be related to a wider infectivity than SARS-CoV. Understanding these dynamic conformational states related to the mechanism by which viruses enter the host cell membrane may lead to the development of effective treatments.
Currently, S protein has been used as an antigen to test DNA vaccines in clinical trials. One of them is called AG0301-COVID19 (ClinicalTrials.gov number, NCT04463472), which used two immunization schedules, the first with a low dose (1.0 mg) and the second with a high dose (2.0 mg). Both injections were injected intramuscularly at two-week intervals. Currently, healthy people between the ages of 20 and 65 are being recruited to evaluate the immunogenicity of the vaccine.
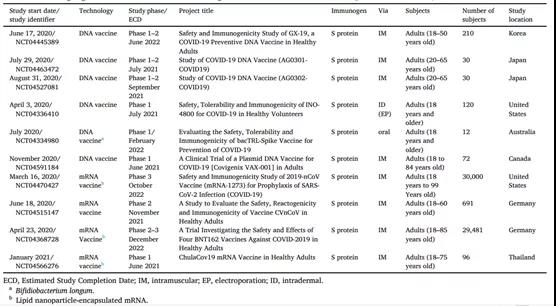
The above table. Overview of ongoing clinical trials for the COVID-19 nucleic acid vaccine (assessed at ClinicalTrials.gov as of November 2, 2020)
The author also mentioned a biotechnology company based in Pennsylvania, USA: INOVIO Pharmaceuticals. This company became one of the first companies to develop DNA vaccines. As early as the first half of last year, the company started the first phase of open label research to evaluate the safety, tolerability and immunogenicity of its DNA vaccine INO-4800. The vaccine is injected intracutaneously by electroporation. In addition, preliminary studies have shown that INO-4800 can induce neutralizing antibodies to block the binding of SARS-CoV-2S protein to the host receptor ACE2.

The author also believes that it is electroporation that uses short electrical pulses at the vaccine application site to increase the permeability of the cell membrane and improve the absorption of antigen; therefore, it can produce a more effective immune response. The author also pointed out that the vaccine carrier has several advantages: low cost, non-invasive administration and high level of safety. The bacTRL-Spike vaccine uses live recombinant Bifidobacterium longum, which contains synthetic plasmid DNA encoding the SARS-CoV-2 S protein. Bifidobacterium is a non-pathogenic anaerobic bacteria, which is part of the human microbiota. In addition, it has been proposed that the bacterium improves the endurance of the host by increasing the immune response to viral infection.
Researchers have also tested some Bifidobacterium strains as antigen carriers for use as recombinant vaccines against hepatitis C virus, enterovirus and cancer. A clinical trial was designed to evaluate the safety and tolerability of the oral bacTRL-Spike vaccine in healthy adults. However, this study has not yet started, and the estimated completion date is December 31, 2021 (ClinicalTrials.gov number, NCT04334980). In addition, the safety and immunogenicity of another DNA vaccine against COVID-19 that uses intramuscular immunization, GX-19, is already in clinical trials.
The challenge
In the current pandemic, several experts have suggested that the immune response to SARS-CoV-2 may lead to antibody-dependent enhancement (ADE). Although this phenomenon is of great significance to flaviviruses and certain cat coronaviruses, SARS-CoV and SARS-CoV-2 have been shown to not cause such effects on living animals or humans.
However, if ADE becomes critical in the current pandemic, both DNA and RNA-based platforms can quickly provide alternatives to circumvent such problems, because the vaccine antigen regions or motifs that cause ADE can be easily engineered Or remove.
The biggest advantage of nucleic acid-based vaccine development technology is that the time from design to clinical trials is very short. Therefore, it may soon be possible to test different variants of antigens covering circulating mutations together in the same vaccine.
This will be an important step in the development of vaccines against rapidly emerging threats, such as the current SARS-CoV-2 pandemic. Therefore, the development of a vaccine against COVID-19 requires further research on genetic mutations and how to avoid vaccine failure due to genetic mutations.
(source:internet, reference only)
Disclaimer of medicaltrend.org



