Innovation in mRNA vaccine technology
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Innovation in mRNA vaccine technology
Innovation in mRNA vaccine technology. In recent years, the most important innovations in mRNA vaccine technology are:
1) mRNA sequence design;
2) development of simple, rapid and large-scale methods for the production of mRNA;
3) development of efficient and safe mRNA vaccine delivery materials.
mRNA sequence design
Considering that the 5’and 3’untranslated regions (UTRs) of mRNA can significantly affect the translation rate and half-life of transcripts, optimizing UTRs is very important for mRNA vaccine design. A recent study used a systemic selection process based on cell culture to identify new UTRs, which significantly increased the protein expression of IVT mRNA. The study identified several 3’UTR sequences. Compared with the human β-globin 3’UTR, these sequences can induce the relevant transcript to produce approximately 3 times the protein. The new 3’UTR motifs have been validated in mRNA vaccination and gene reprogramming studies, and they induce a more effective therapeutic effect than the mRNA of β-globin 3’UTR.
In addition, an interesting new vaccine form has recently been reported, showing great efficacy without delivery materials. This method uses the mRNA encoding the alpha virus RNA-dependent RNA polymerase plus the second mRNA encoding the antigen to enable it to replicate in the cytoplasm. This system can effectively induce a protective immune response in mice at a very low dose (50ng). These findings are particularly attractive because the use of low doses reduces the cost of vaccine production. The absence of delivery materials further reduces costs, simplifies manufacturing, and increases the possibility of vaccine freeze-drying and storage at ambient temperature.
Optimization of mRNA production
The development of rapid, simple, large-scale and inexpensive methods to produce high-quality mRNA is a key requirement for the implementation of mRNA vaccines in the future. Two recent innovations have made progress in this regard: The first is a co-transcription capping technology called CleanCap@, which adds a natural 5’cap1 structure to a specific transcription initiation sequence during the IVT process .
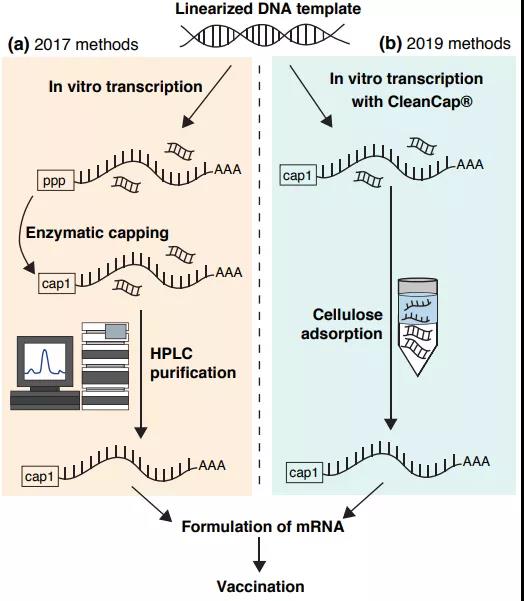
This progress is significant because previous research schemes used enzymatic methods and added additional reaction components and purification steps in the manufacturing process. When IVT mRNA preparation contains dsRNA contaminants, innate immune activation will occur.
The second innovation provides an attractive alternative to high-performance liquid chromatography purification, which can be used in laboratory and industrial-scale production. This is a simple method to purify mRNA by adsorbing double-stranded RNA contaminants to cellulose, a cheap and abundant polysaccharide. Studies have proved that this highly scalable and inexpensive method is as effective as high-performance liquid chromatography for removing dsRNA contaminants in IVT mRNA samples.
Development of high-efficiency in vivo mRNA delivery materials
The most important recent progress in the field of mRNA vaccines has been made in this field, and has played a key role in advancing the application of mRNA vaccines.
polymer
Recently, a new type of lipid-containing polymer has been developed, called Changed Charge Releasable Transporters (CARTs), which can effectively target T cells and effectively eliminate established tumors in mice. Manipulating T cells is very difficult and usually requires in vitro operations (purification of T cells obtained from donors, nucleic acid electroporation, amplification and refusion). Therefore, CART is an attractive delivery material for mRNA vaccines. And the field of gene therapy has great potential.
Another branched polyamine-based polymer composed of lipid-anchored polyethylene glycol (PEG) and antigen-encoded and self-amplified mRNA is called dendrimer. A single intramuscular injection can induce antigen-specific CD8+ T cell responses and neutralizing antibodies to Zika, Ebola, influenza virus and Toxoplasma gondii in mice.
Peptides
Cell penetrating peptides (CPPs) are rarely used in mRNA vaccines, but there have been some recent advances in this area: CPPs containing amphiphilic Arg-Ala-Leu-Ala motifs have been developed to condense mRNA into a form that can destroy and penetrate Membrane-permeable particles, and after immunization of mice, show a powerful killer T cell response.
Lipid nanoparticles
Lipid nanoparticles (LNPs) are currently the most widely used mRNA carrier materials, and several clinical trials using mRNA LNP are currently underway (NCT03076385 and NCT0334343). In these experiments, the nucleoside modified mRNA-LNP influenza vaccine induced a humoral immune response in the subjects, and the safety of the vaccine was comparable to that of the inactivated influenza vaccine. Compared with preclinical studies, the degree and durability of the immune response are relatively small and need to be improved.
It is worth noting that the above-mentioned clinical trials started a few years ago, and there are currently more effective LNP preparations. Similar to the polymer-based CART platform, mRNA-LNPs also achieve selective T cell targeting. A new platform, called ASSET (anchored secondary targeting single chain antibody), in which T cell-specific monoclonal antibodies are linked to LNP to target T cells. This flexible platform also has great potential in mRNA vaccines and other applications.
Another lipid complex preferentially targets dendritic cells after systemic administration. The use of mRNA vaccines to selectively target DCs to induce a strong immune response is a potentially key discovery, and the platform has proven its promise in clinical trials.
Summary:
In the past few years, extremely important progress has been made in the field of mRNA vaccines, and has provided evidence for the feasibility of this new vaccine model. New manufacturing methods and delivery materials will facilitate the rapid and inexpensive mass production of next-generation mRNA vaccines.
The data from human cancer and infectious disease mRNA vaccine trials are encouraging, but there are also many areas that need in-depth research, including reducing adverse reactions after vaccine injection, and a more comprehensive understanding of the mechanism of action of various mRNA vaccine types to further Optimize mRNA vaccine technology.
(source:internet, reference only)
Disclaimer of medicaltrend.org



