Three doses of Moderna COVD-19 vaccine better against B.1.351 infections
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Three doses of Moderna COVD-19 vaccine better against B.1.351 infections
Three doses of Moderna COVD-19 vaccine better against B.1.351 infections. The effectiveness of existing vaccines to prevent COVID-19 mutant strains has been significantly reduced; Moderna has made another move and an additional booster vaccination is enough.
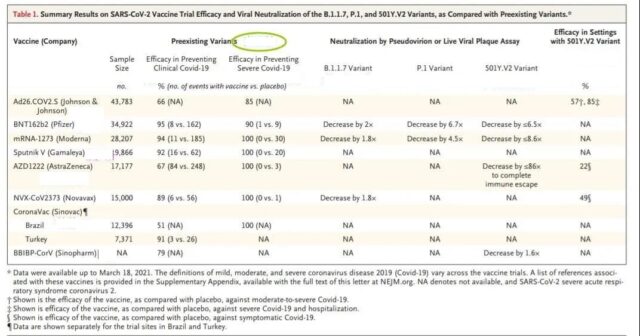
A series of recent studies have shown that the new coronavirus continues to mutate, resulting in reduced or even loss of protection of the originally developed vaccine. Among them, the immune escape of the South African mutant is the most obvious.
On March 25, 2021, South African scientists published an article in the New England Journal of Medicine (NEJM), summarizing the characteristics of the current three major variants of the new coronavirus and the protection of the current seven major vaccines.
The researchers demonstrated the neutralizing activity of the vaccinated sera against different mutant strains in laboratory studies, showing that the vaccinators of Pfizer, Moderna, AstraZeneca and Sinopharm had reduced neutralizing activity against the mutants. But the most obvious reduction was that the neutralizing activity of the AstraZeneca vaccinators against the South African mutant (501Y.V2) was reduced by 86 times.
The results of the clinical trials of the vaccine against the 501Y.V2 variant in South Africa summarized by the researchers are also not optimistic, and they are significantly lower than the protection of the vaccine against non-mutant strains; among them, the AstraZeneca vaccine is nearly ineffective.
On March 12, 2021, Cell magazine published an article by the Ragon Institute and the Balazs group of MGH. This study is currently the most comprehensive one to evaluate the changes in serum neutralization activity against various SARS-CoV-2 mutant strains after mRNA vaccination. Research.
The study found that after two inoculations with BNT162b2 or mRNA-1273, the neutralizing activity of the inoculated plasma against the British mutant B.1.1.7 decreased, but not significantly; the neutralizing activity against the Brazilian mutant P.1 decreased to a certain extent, but it was The South African mutant B.1.351 decreased most significantly.
It is imperative to develop new vaccines against mutant strains
The American company Moderna uploaded on bioRxiv the partial results of a phase II clinical trial of its vaccine mRNA-1273.351 against the South African mutant strain B.1.351, and issued a statement on May 5.
The study found that an additional 50µg of mRNA-1273 or mRNA-1273.351 given to people who had previously been vaccinated with Moderna vaccine can significantly increase the neutralizing activity of the vaccinated against the South African mutant strain B.1.351 and the Brazilian mutant strain P.1. This study evaluated 3 strategies: 1. Give an additional mRNA-1273.351 to the previous vaccinated person; 2. Give the previous vaccinated mRNA-1273.211, which is a 1:1 mixture of mRNA-1273.351 and mRNA-1273; 3. mRNA-1273.351 Primary immunity + mRNA-1273 enhancement.
The study conducted a neutralization test (PsVN) to test the neutralization titer of their serum about 6 to 8 months after the initial vaccination of the mRNA vaccine. Among 40 subjects, 37 subjects could detect serum neutralizing activity, but subjects had much lower neutralizing activity against VOC mutant strains (B.1.351 and P.1), and about half of them were affected. The subject’s titration is below the detection limit. Two weeks after receiving mRNA-1273 or mRNA-1273.351 booster vaccination, serum PsVN titers of all subjects were significantly increased. After the booster immunization, the neutralizing activity GMT of the B.1.351 and P.1 mutant strains against the wild-type strain increased to a level or higher than the previously reported peak titer after the initial vaccination against the wild-type strain (D614G).
Compared with mRNA-1273, the neutralizing activity of mRNA-1273.351 against the B.1.351 mutant strain is more significant, and the subjects’ serum GMT levels are higher 15 days after the booster vaccination, where mRNA-1273.351, GMT = 1400; mRNA- 1273, GMT = 864. Before the booster vaccination, the neutralizing activity of the subjects against wild-type D614G and B.1.351 differed by 7.7 times; after booster vaccination with mRNA-1273.351, the difference was only 2.6 times.
In earlier preclinical experiments, researchers found that various strains including B.1.351 in serum after immunization with mRNA-1273.351 have broad-spectrum neutralizing activity.
In addition, the study also suggested that on the basis of two previous immunizations with common mRNA-1273, an additional mRNA-1273.351 can prevent both wild-type and B.1.351 infections.
Editor’s note:
The coronavirus is constantly undergoing mutations. Is the original vaccine useless?
Moderna’s research shows that on the basis of the original vaccination, an additional mRNA-1273.351 can prevent both wild-type and B.1.351 infections.
(source:internet, reference only)
Disclaimer of medicaltrend.org



