COVID-19 Vaccine Company: Cansino obtained EU GMP certification
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
China COVID-19 Vaccine Company: Cansino obtained EU GMP certification
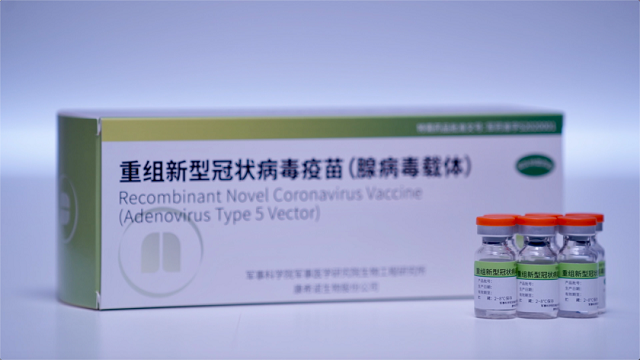
COVID-19 Vaccine Company: Cansino obtained EU GMP certification. Cansino Biotech is the vaccine company to make various vaccines including Ebola and COVID-19 vaccine. Their COVID-19 vaccines are adenovirus vector vaccines, which use the similar technology as AstraZeneca COVID-19 vaccines.
Cansino Biotech Co., Ltd. (hereinafter referred to as “Cansino Bio”, Cansino-U 688185.SH, Cansino Bio-B06185.HK) was awarded the Hungarian National Institute of Pharmacy and Nutrition on May 21. , issued the EU GMP certificate for recombinant new coronavirus vaccine (adenovirus vector type 5) (Ad5-nCoV), becoming the first new coronavirus vaccine manufacturer in China to obtain GMP certification in the EU for innovative vaccine technology routes.

This means that CanSino Bio already has a commercial production base and quality management system that meets EU standards, which has laid a solid foundation for the company’s products to enter the international market.
On January 13, the Hungarian National Institute of Medicine and Nutrition conducted a GMP review of Cansino Bio, and determined that Cansino Bio complies with EU GMP standards and guidelines. On May 21, it officially issued the EU General GMP Certificate to Cansino Bio. .
Up to now, CanSinoBio’s recombinant new coronavirus vaccine (type 5 adenovirus vector) KVisa has been approved for conditional marketing in China, and has obtained emergency use authorization in Mexico, Pakistan, Hungary, and Chile. He is 18 years old in the local area. Adults and above provide fast and effective immune protection, while meeting the domestic demand for COVID-19 vaccination, and helping the world to build an immune barrier.
About CanSino Bio
Cansino Biotech Co., Ltd. (H-share abbreviation: Cansino-B, code 06185.HK; A-share abbreviation: Cansino-U, code 688185.SH), established in Tianjin, China in 2009, is committed to serving the Chinese and global public Health research and development, production and commercialization of innovative vaccines.
The company currently has four innovative vaccine platform technologies, including adenovirus vector vaccine technology, binding technology, protein design and recombination technology, and preparation technology. At present, the company has established a strong R&D pipeline of 16 vaccines covering 13 infectious diseases, including the global innovative recombinant Ebola virus disease vaccine (adenovirus vector) approved in 2017 and the recombinant new coronavirus vaccine (adenovirus vector) ).
(source:internet, reference only)
Disclaimer of medicaltrend.org



