CanSino released Phase 1 data of nebulized inhalation COVID-19 vaccine
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
CanSino released Phase 1 data of nebulized inhalation COVID-19 vaccine
CanSino released Phase 1 data of nebulized inhalation COVID-19 vaccine. No injections, lower doses! Nebulized inhalation COVID-19 Vaccine developed by Academician Chen Wei/Cansino: Phase 1 Clinical Results Announced.
On July 27th, the preliminary report of Phase 1 clinical trial of the nebulized inhalation vaccination adenovirus vector type 5 neocorona vaccine (Ad5-nCoV) jointly developed by the team of Academician Chen Wei of the Chinese Academy of Engineering and Cansino Biology was published in The Lancet-Infectious Diseases. Published.
The results of the study
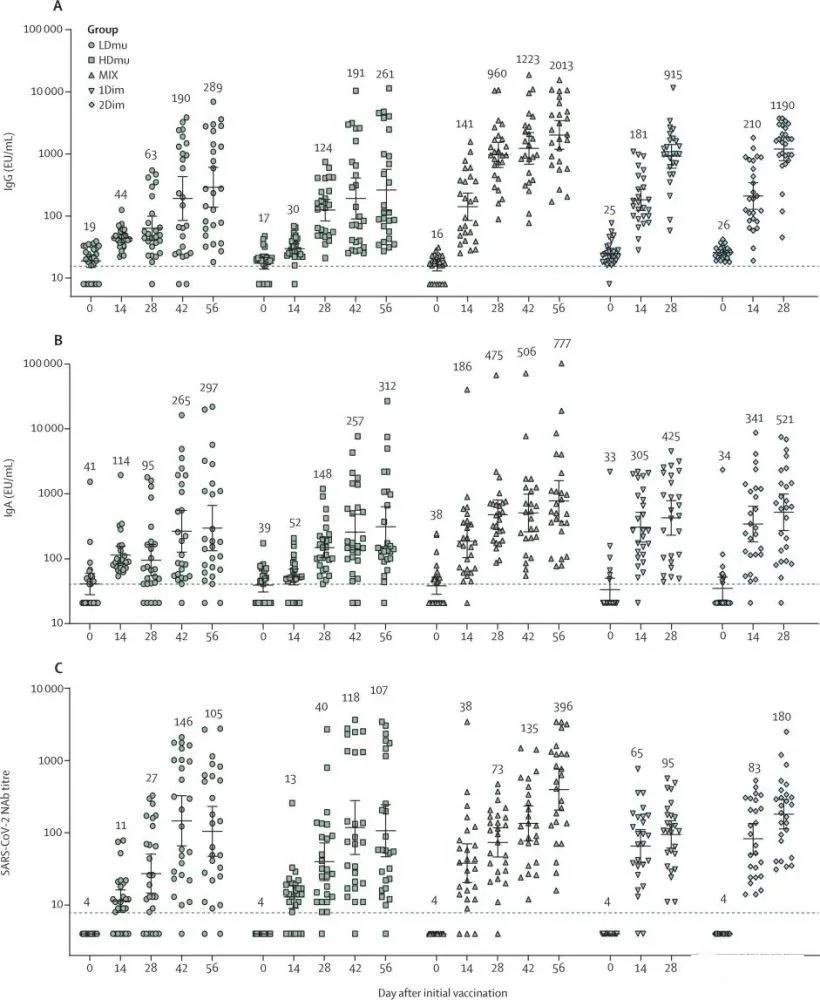
The results showed that Ad5-nCoV was well tolerated by nebulized inhalation and did not cause any serious adverse events related to the vaccine. One dose of aerosolized Ad5-nCoV is equivalent to one-fifth of the dose of a single intramuscular injection. Nebulized inhalation of two doses of Ad5-nCoV elicits a new coronavirus neutralizing antibody response similar to that of a single intramuscular injection of the vaccine. On the other hand, aerosol inhalation booster immunity on the 28th day after intramuscular injection of Ad5-nCoV can induce a strong neocoronavirus-specific IgG antibody and neutralizing antibody response.

This is a randomized, open phase 1 clinical trial to evaluate the safety and immunogenicity of nebulized Ad5-nCoV vaccine in Chinese healthy adults (≥18 years of age). In this test, there was a moderate correlation between RBD-specific IgG antibody titers and neutralizing antibody titers. Neocorona neutralizing antibodies are not only derived from RBD-related antibodies, but also from N-terminal domain-related antibodies of S protein, which may partly explain the difference in seroconversion rates between RBD-specific IgG antibodies and neutralizing antibodies.
At the same time, the article points out: the inhalation dose of a single dose is equivalent to one-fifth or two-fifths of the usual single-dose intramuscular injection.
After the Ad5-nCoV vaccine is vaccinated by different routes, the concentration of IgG antibody (A) and IgA antibody (B), and the level of neutralizing antibody (C) are measured. (Two doses of high-dose aerosol inhalation: HDmu, two doses of low-dose aerosol inhalation: LDmu, mixed immunity: MIX, single dose intramuscular injection: 1 Dim, two doses a day intramuscular injection: 2 Dim)
At the same time as the picture, the article points out: the inhalation dose of a spray is equivalent to one-fifth or two-fifths of the usual single-dose intramuscular injection.
End
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



