First Phase III clinical data of COVID-19 inactivated vaccine is released!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The world’s first Phase III clinical data of COVID-19 inactivated vaccine is officially released!
First Phase III clinical data of COVID-19 inactivated vaccine is released! On May 26, the international medical journal “The Journal of the American Medical Association” (JAMA) published the “Protection of Two Novel Coronavirus Inactivated Vaccines for Adults from COVID-19 Infection” from China SINOPHARM Biotech.
Effectiveness Evaluation” (“Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults”). The report summarizes and analyzes the effectiveness and safety of the Beijing Institute of Biological Products (BIBP) and Wuhan Institute of Biological Products (WIBP) of the COVID-19 inactivated vaccines in Phase III based on the results of the Phase III clinical trial of the China Biologics COVID-19 Inactivated Vaccine Clinical trial results.
This si the world’s first officially published Phase III clinical trial results of a COVID-19 vaccine inactivated, and this is the first time that China’s COVID-19 vaccine Phase III clinical trial results have been published.
The report was prepared by Sinopharm China Biotechnology, National Joint Vaccine Engineering Technology Research Center, Wuhan Institute of Biological Products, Beijing Institute of Biological Products, Abu Dhabi Health Services Company (SEHA) Sheikh Khalifa Medical City, Kingdom of Bahrain Military Hospital , United Arab Emirates Ministry of Health and Prevention, Prince Hamza Hospital in Amman, Jordan, Egyptian Ministry of Health, Henan Provincial Center for Disease Control and Prevention, Beijing Kete Statistical Consulting Co., Ltd., Huazhong University of Science and Technology, G42 and many other institutions and units.
Corresponding author The joint prevention and control mechanism of the State Council Science and Technology Expert Group, the director of the National Joint Vaccine Engineering Technology Research Center, the chief scientist of the “863” program vaccine project of the Ministry of Science and Technology, and Yang Xiaoming, chairman of Sinopharm Group China Biotechnology, etc. The report was submitted to “JAMA” for review and review on March 17, 2021.
▲The Journal of the American Medical Association (JAMA), which was founded in Chicago, USA on July 14, 1883, has been published continuously for 138 years. It is one of the most famous medical journals in the world. One. The purpose of JAMA is to promote the improvement of medical science, medical technology and public health.
Highlights Summary
The results of this study are based on an interim analysis conducted by a randomized, double-blind, placebo-controlled phase III clinical trial of two COVID-19 inactivated vaccines from China Biologics.
Since July 16, 2020, 40,411 subjects were enrolled and randomly divided into three groups, namely the aluminum adjuvant group, the COVID-19 vaccine WIV04 group and the COVID-19 vaccine HB02 group.
The subjects received two intramuscular injections on day 0 and day 21. 14 days after the second dose of vaccination, a total of 142 effective cases were confirmed (95 in the aluminum adjuvant group, 26 in the WIV04 group, and 21 in the HB02 group). The effective rates of the two vaccine groups were 72.8% and 78.1%
Research method
This study is a randomized, double-blind, placebo-controlled phase III clinical trial. The Beijing Institute of Biological Products (BIBP) and Wuhan Institute of Biological Products (WIBP) affiliated to China National Biological Technology Co., Ltd. are the sponsors, and the participating investigators will implement and To collect relevant data, they came from Abu Dhabi Sheikh Khalifa Medical City, Al Qarain Health Center in Sharjah, Salmanyia Hospital in Bahrain, Vacsera Medical Center and Katameya Medical Center in Egypt, and Prince Hamza Hospital in Jordan.
In addition, an independent data and safety monitoring committee (DSMB) was established to monitor safety data and assess the risks of subjects. The clinical protocol was approved by the institutional review board of each participating country (Abu Dhabi Health COVID-19 Research Ethics Committee, UAE Ministry of Health and Prevention Research Ethics Committee, Bahrain National Health Authority Independent Research Ethics Committee, Jordan Prince Hamza Hospital Institutional Review Board and the Research Ethics Committee of the Research and Health Development Bureau of the Ministry of Health and Population of Egypt). All subjects signed an informed consent form before enrollment.
The subject is a population of 18 years and older, and has no previous history of SARS-CoV or new coronavirus or MERS infection (on-site inquiry). Each subject was randomly assigned a study number generated by an independent statistician (each vaccine/placebo has a unique number), and randomly assigned to the placebo group containing only aluminum hydroxide (aluminum) adjuvant or Any one of two candidate vaccines (1:1:1 ratio). The subjects received two intramuscular injections 21 days apart.
The two vaccines WIV04 and HB02 in this clinical study were developed and produced by Wuhan Institute of Biological Products and Beijing Institute of Biological Products. The strains used (WIV04 and HB02) were isolated from two patients in Wuhan Jinyintan Hospital. The virus strain was cultured and propagated in a qualified Vero cell line from WHO, followed by inactivation and purification. All vaccines and placebos are verified by the China Institute for Food and Drug Control. After the blinding, each vaccine is used in a single-dose vial with a unique code and the same appearance.
In the study, subjects were informed of the COVID-19 and related symptoms, and case monitoring was carried out through a combination of active follow-up by the investigator (by phone calls every week), active reports from subjects and active reports from sentinel hospitals. If the subject has one or more of the following three symptoms, it is defined as a suspected COVID-19 case:
1) At least two of the following symptoms last for at least 2 days: fever (axillary temperature ≥37.5°C), chills, sore throat, nasal congestion, muscle pain, fatigue, headache, nausea or vomiting, diarrhea;
2) At least one respiratory system sign or symptom (including cough, shortness of breath), or new smell or taste disorder, or shortness of breath;
3) Clinical or imaging evidence of pneumonia.
Suspected cases with new coronavirus infection-like symptoms are required to go to a designated hospital for laboratory testing immediately, including real-time reverse transcription polymerase chain reaction (RT-PCR) testing of nasal or nasopharyngeal swab specimens and serum specimen-specific IgG antibody testing. If the patient is tested positive for the new coronavirus nucleic acid by RT-PCR, it is a laboratory confirmed case. Subsequently, the case is submitted to the endpoint determination committee for case confirmation and determination of the severity of the case.
Research results:
This project screened 46270 subjects, 40411 subjects were randomly assigned to aluminum adjuvant group (13471), WIV04 group (13470), HB02 group (13470). In the end, 40,382 subjects completed the first injection: 13,458 in the aluminum adjuvant group, 13,459 in the WIV04 group, and 13,465 in the HB02 group; 39,223 subjects completed the second injection, with 13,071 in each group. , 13066 people and 13,086 people.
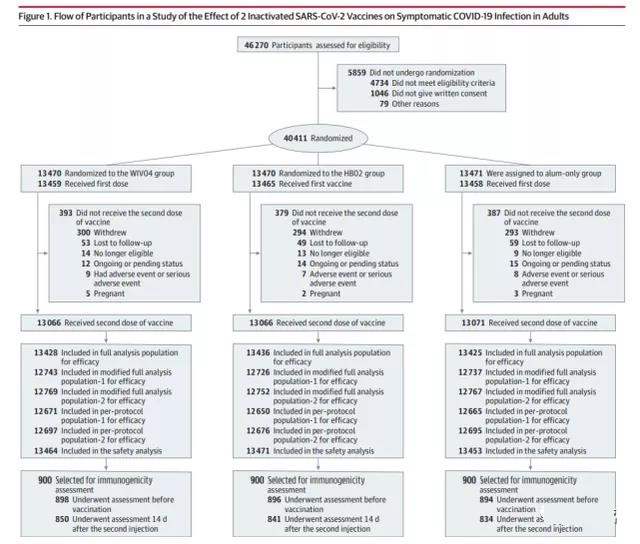
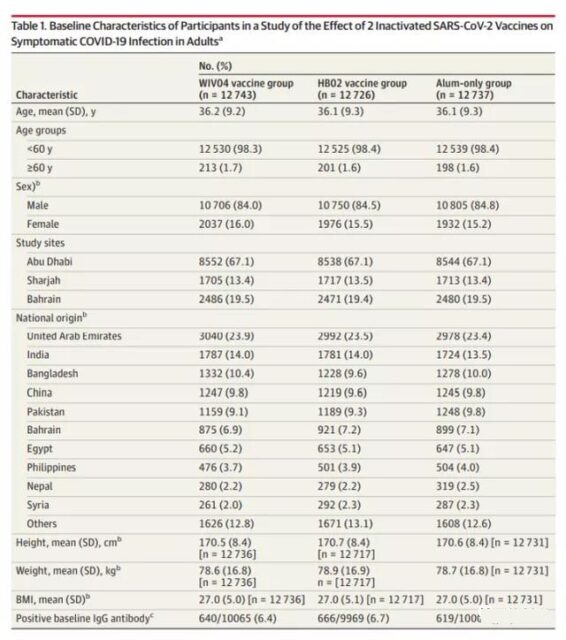
The average age of the subjects in the project was 36.1 years old, and 84.4% were men. 25,634 (67.1%) were from Abu Dhabi, UAE, 5,135 (13.4%) were from Sharjah, UAE, and 7,437 (19.5%) were from Bahrain. There were no significant differences in all characteristics among the groups.
As of December 20, 2020, there have been 962 suspected cases after the first injection. After review by the Endpoint Judgment Committee, a total of 255 confirmed cases were obtained, of which 142 confirmed cases were in the monitoring period (14 days after the second injection), and 113 confirmed cases were outside the monitoring period (the first injection to the 35th day) ).
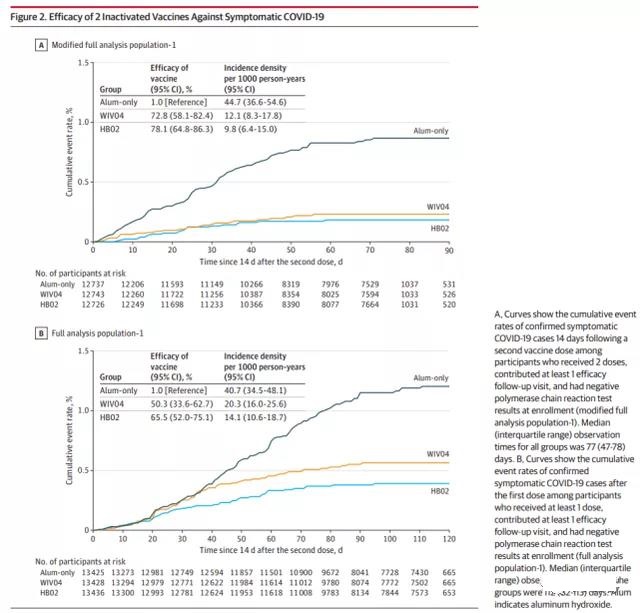
Among the 142 cases during the monitoring period, 95 were in the aluminum adjuvant group, 26 were in the WIV04 group, and 21 were in the HB02 group. Compared with the aluminum adjuvant group, the protective effect of the WIV04 group was 72.8% (95% CI, 58.1%-82.4%), and the protective effect of the HB02 group was 78.1% (95% CI, 64.8%-86.3%).
A total of 47 asymptomatic cases were detected 14 days after the second injection: 21 in the aluminum adjuvant group, 16 in the WIV04 group, and 10 in the HB02 group. After these asymptomatic cases are included in the main analysis set, the estimated protective efficacy of the WIV04 vaccine is 64.0% (95% CI, 48.8%-74.7%), and the estimated protective efficacy of the HB02 vaccine is 73.5% (95% CI, 60.6 %-82.2%).
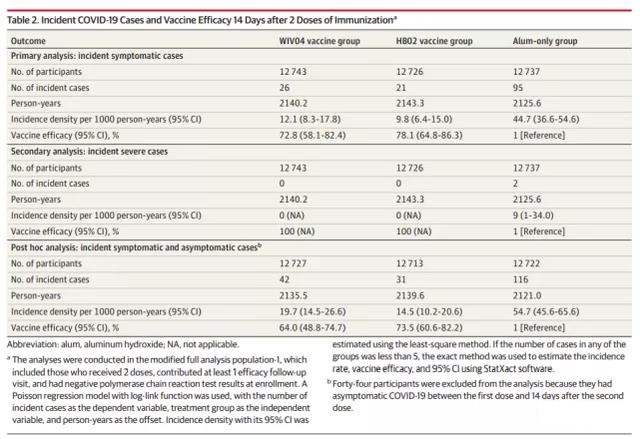
Within 35 days of the first injection (outside the monitoring period), a total of 113 confirmed cases were detected: 43 in the aluminum adjuvant group, 43 in the WIV04 group, and 27 in the HB02 group. The results showed that the protective efficacy of the WIV04 vaccine against confirmed COVID-19 cases after the first injection was 50.3% (95% CI, 33.6%-62.7%), and the HB02 vaccine was 65.5% (95% CI, 52.0%-75.1%). Before the 21st day (the date of the second injection), the incidence of the aluminum adjuvant group was similar to that of the HB02 group and the WIV04 group. After the second dose, the incidence of the vaccine group was significantly lower than that of the aluminum adjuvant group.
Among the collected cases, only 2 severe cases occurred in the aluminum adjuvant group, and no severe cases occurred in the two vaccine groups, so the vaccine group has a 100% protective effect on severe cases of COVID-19 pneumonia. However, given the small number of severe cases, the results have yet to be collected. There were no deaths during the entire follow-up period.
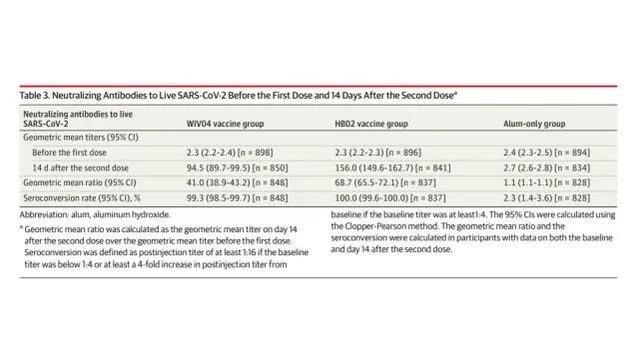
In the immunogenicity study, the geometric mean titers (GMTs) of neutralizing antibodies at baseline were 2.3 in both vaccine groups, 2.4 in the aluminum adjuvant group, and GMTs at 14 days after the second dose was 94.5 in the WIV04 group ( 95% CI, 89.7-99.5), 156.0 (95% CI, 149.6-162.7) in the HB02 group, and 2.7 (95% CI, 2.6-2.8) in the aluminum adjuvant group. The seroconversion rate (4-fold increase) of WIV04 group, HB02 group and aluminum adjuvant group were 99.3%, 100.0% and 2.3%, respectively.
Safety aspect
Within 7 days after each dose, 5957 people (44.2%) in the WIV04 group, 5623 (41.7%) in the HB02 group, and 6250 (46.5%) in the aluminum adjuvant group reported adverse events. The most common adverse event reported by the three groups was injection site pain (24.3%, 19.4%, 27.9%), followed by headache (12.9%, 13.1%, 12.6%). Most adverse events are mild (grade 1 or 2), transient and self-limiting, and do not require special treatment. 8-28 days after vaccination, the incidence of non-collective adverse events was 16.1%, 15.5%, and 15.4% in the WIV04 group, HB02 group, and aluminum adjuvant group, respectively.
Non-collection adverse events (injection-related or unrelated), the incidence of non-collection adverse events were 48.3%, 46.1%, and 50.5% in the WIV04 group, HB02 group, and aluminum adjuvant group, respectively. During the follow-up period, there were 201 serious adverse events (SAE) (Grade 3 or above). The incidence of the three groups was 0.5% in the WIV04 group, 0.4% in the HB02 group, and 0.6% in the aluminum adjuvant group. There was no significant difference between the three groups.
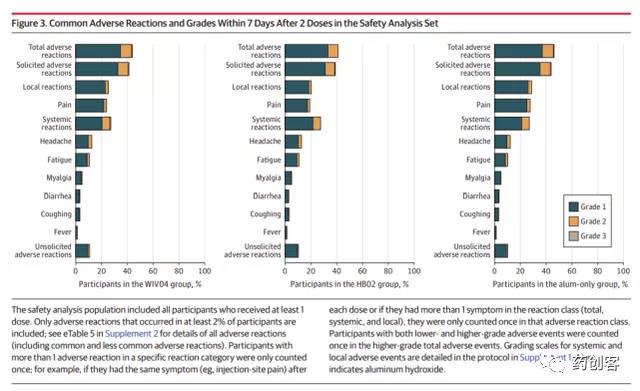
The results of the study show that the two new inactivated vaccines of China Biologics can produce high-titer antibodies and form effective protection 14 days after two injections, and the positive conversion rate of neutralizing antibodies in the whole population has reached more than 99%. The protective efficacy of the WIV04 vaccine group was 72.8%, and that of the HB02 vaccine group was 78.1%. The safety is good, and the adverse reactions are mostly pain at the injection site, which is mild, transient and self-limiting.
(source:internet, reference only)
Disclaimer of medicaltrend.org



