Pfizer released Phase III data of COVID-19 vaccines
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Pfizer released Phase III data of COVID-19 vaccines
Pfizer released Phase III data of COVID-19 vaccines. On December 10th, Pfizer and BioNTech’s COVID-19 vaccine team published the results of phase III clinical trials on NEJM. This is the world’s first new coronavirus vaccine to announce detailed data on phase III trials.
A total of 43548 subjects were enrolled in the phase III trial. The subjects were divided into two groups and received the COVID-19 vaccine and placebo on day 0 and day 21 of the trial.
As with the previous phase I and II results, the most common local adverse reaction is still pain at the injection site, and less than 1% of the vaccine group participants reported severe pain. Moreover, among people over 55 years of age, the incidence of pain at the injection site is lower than that of people aged 16 to 55.
Other local adverse reactions include redness and swelling at the injection site. These local adverse reactions can be relatively mild, and they can subside on their own after 1 to 2 days of vaccine injection.
For systemic adverse reactions, the probability of occurrence in the age group over 55 years is also lower than that of subjects between 16 and 55 years old. In addition, the probability of systemic adverse reactions after the second injection on the 21st day is higher than that after the first injection.
After the first injection, the incidence of severe systemic adverse reactions was less than 0.9%. After the second injection, except for fatigue (3.8%) and headache (2.0%), the incidence of more severe systemic adverse reactions was less than 2%.
Among them, the most common systemic adverse reactions are fatigue and headache, which are similar to the results of the previous phases I and II. The probability of fatigue and headache in the age group over 55 years old is 51% and 39%, respectively. These two figures are lower than those of subjects aged 16 to 55 (fatigue 59% and headache 52%).
16% of the young subjects and 11% of the older subjects developed fever after the second shot of the vaccine, and 2 subjects developed a fever above 40°C. These fever symptoms all appear within 48 hours after injection, and can be relieved after symptomatic treatment.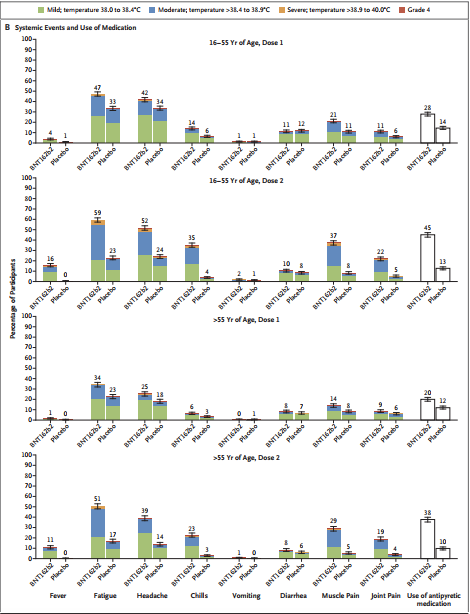
Source: Reference 1
During the entire phase III trial, 2 subjects in the vaccine group unfortunately passed away. Looking back at the causes of death, one of them was due to cardiac arrest, and the other was due to coronary heart disease due to atherosclerosis. At present, experimenters believe that the two deaths are not specifically related to vaccination.
Recently, 4 cases of Bell Palsy (Bell Palsy) occurred in subjects vaccinated with this vaccine. This matter has aroused widespread concern in the medical community and society. However, this symptom did not appear as an adverse reaction alone in the NEJM article.
As early as November 18, Pfizer and BioNTech announced on the official website that the vaccine has an effective rate of 95%. This result is further analyzed in the article just published.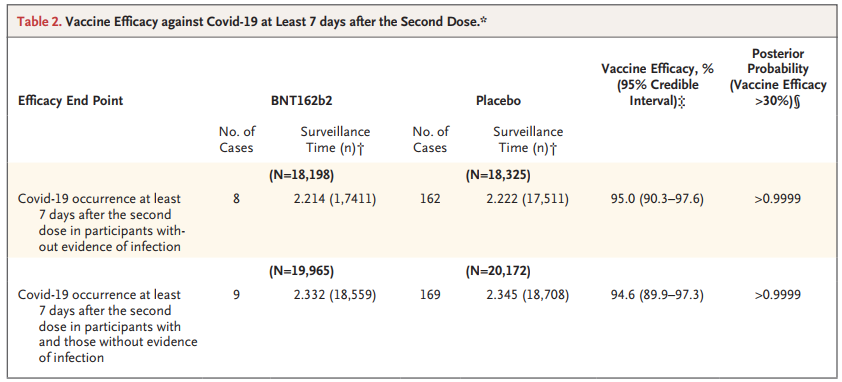
Source: Reference 1
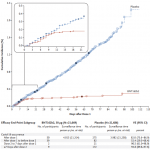
At least 7 days after the second injection of the vaccine, there are currently 8 confirmed cases of the new coronavirus in the vaccine group and 162 cases in the control group. After statistical calculation, the effective rate of this vaccine is 95.0% (confidence interval 90.3-97.6%).
Between the first shot of the vaccine and the second shot of the vaccine, there were 39 confirmed cases of the new coronavirus in the vaccine group, while 82 cases were confirmed in the control group. Between the two injections, the vaccine’s effective rate was 52% (confidence interval 29.5-68.4%).
Existing data believe that 12 days after the first injection of the vaccine, the vaccine can provide a certain degree of protection. However, comparing the 52% effective rate between the two injections and the 95% effective rate one week after the second injection, it is still There is a huge gap.
Source: Reference 1
During the entire phase III clinical trial, a total of 9 cases of severe COVID-19 pneumonia occurred in the control group and 1 case in the vaccine group. The experimenters believe that this difference in data shows that the vaccine can effectively prevent the aggravation of COVID-19 pneumonia symptoms.
During the development of the COVID-19 vaccine, some virologists are very worried that the vaccine will cause Vaccine-mediated Disease Enhancement.
This is a potential side effect of a vaccine. It means that the neutralizing antibodies in the body not only cannot prevent the virus from invading, but also cause the body to produce more serious symptoms after being infected with the pathogen.
But judging from the current data provided by Pfizer Vaccine, relevant scholars can temporarily relax their minds on this issue.
There are also some limitations in the current phase III trial data.
First of all, the current follow-up time after the second injection is two months. This time is still slightly insufficient for the detection and effectiveness evaluation of the vaccine’s adverse reactions. This requires a longer follow-up of subjects.
On the other hand, the current article only analyzes the situation of volunteers over the age of 16 after being injected with the vaccine. There is still a lack of data to support the safety and effectiveness of the vaccine for adolescents, pregnant women, and immunodeficiency patients.
Disclaimer of medicaltrend.org



