SINOVAC released trial data of 3rd-dose (booster) of COVID-19 vaccine
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
SINOVAC released trial data of 3rd-dose (booster) of COVID-19 vaccine
SINOVAC released trial data of 3rd-dose(booster) of COVID-19 vaccine. SINOVAC(Kexing) released the first clinical trial results of the COVID-19 vaccine booster injection.
On July 25, 2021, the Vaccine Evaluation Institute of Jiangsu Provincial Center for Disease Control and Prevention, the School of Public Health of Fudan University, Kexing Holding Biotechnology Co., Ltd. (SINOVAC Kexing), Huashan Hospital Affiliated to Fudan University, etc. The research team published a paper on Medrxiv with below title:
“Immunogenicity and safety of a third dose, and immune persistence of CoronaVac vaccine in healthy adults aged 18-59 years: interim results from a double-blind, randomized, placebo-controlled phase 2 clinical trial”
The research results mainly include safety and immunogenicity
Safety
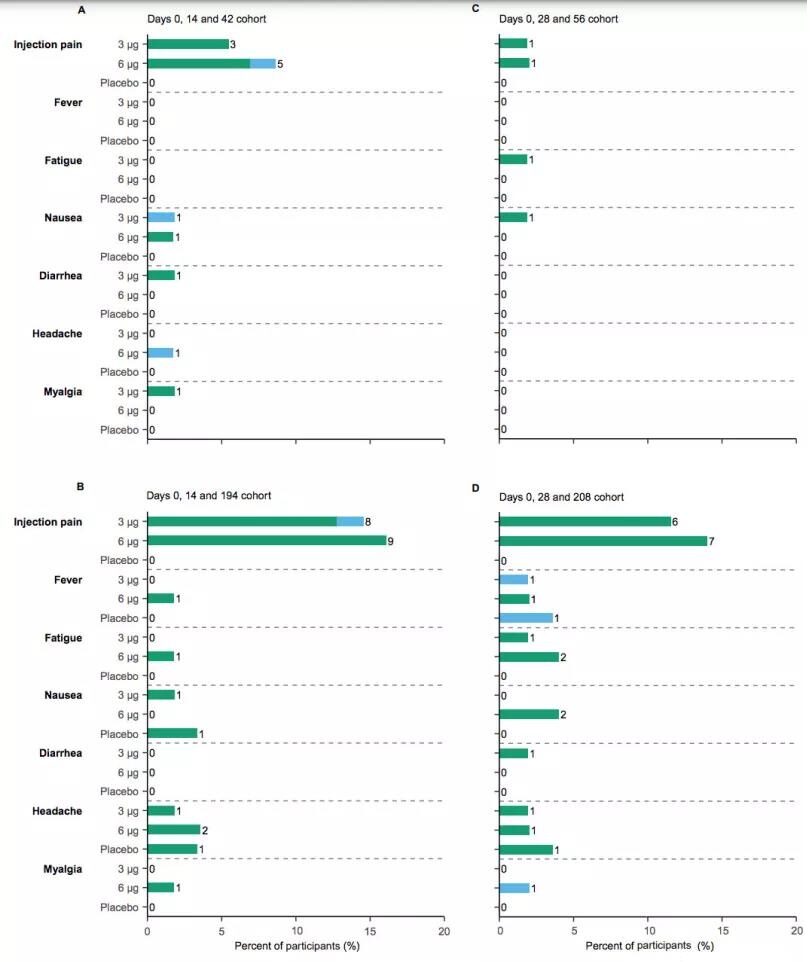
The overall safety is good, the most common is the injection point pain, the probability of occurrence after the third injection in the first and third groups is 7.91% and 3.08%, which is lower than the overall occurrence probability of the adverse reaction in these two groups ( 33.33% and 22.00%).
In the 3ug group, the first two needles were separated by 14 or 28 days, and the third needle was separated by 6 months. The incidence of adverse reactions in the third needle was basically the same. The second and fourth groups were 18.18% and 19.23%, respectively. A total of 14 serious adverse reactions were reported by 9 volunteers, and no vaccine-related events were found after research.
Immunogenicity
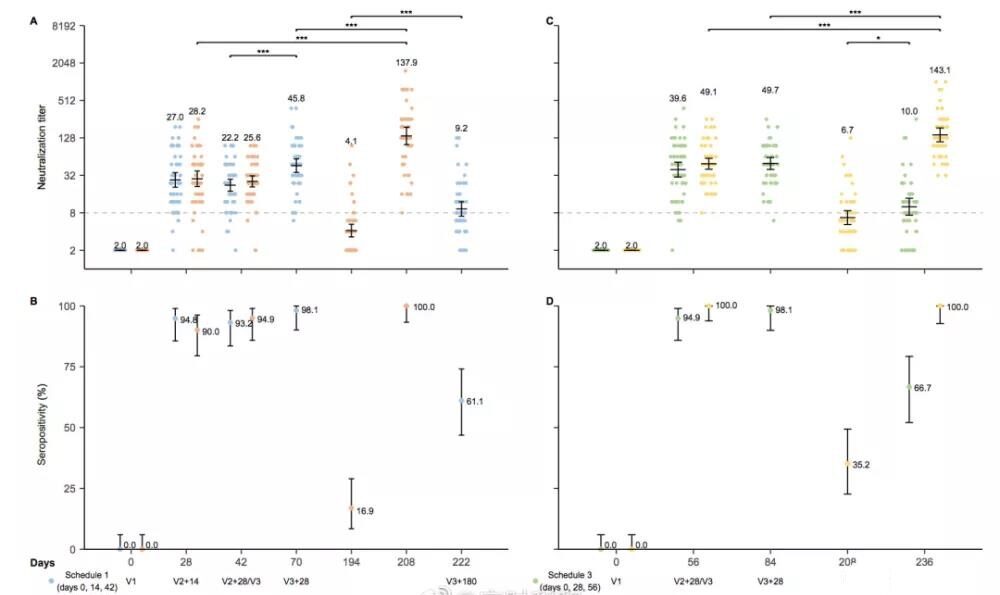
1. An interval of 28 days between the first two injections will produce a higher neutralizing antibody GMT than an interval of 14 days.
Combining the data of the first and second groups and the third and fourth groups,
- The positive conversion rate of the antibody after 14 days and 28 days after the second injection is 93.2%-94.9%, and the neutralizing antibody GMT is 22.2-25.6;
- The positive conversion rate of the antibody after 28 days and 28 days after the second injection is 94.9%~100%, and the neutralizing antibody GMT is 39.6~49.1.
2. With only two injections, the antibody level will be greatly reduced after half a year.
- In the second group where the first two injections are 14 days apart, on day 194 (before the third injection), the antibody positive conversion rate is only 16.9%, and the neutralizing antibody GMT is only 4.1;
- The fourth group with 28 days interval between the first two injections is not much better after half a year. The antibody positive conversion rate is only 35.2%, and the neutralizing antibody GMT is only 6.7.
3. The third dose of vaccination is effective and effectively induces high levels of neutralizing antibodies.
- On the 28th day after the third injection of the first group (0-14-42), the neutralizing antibody GMT reached 45.6, but it fell to 9.2 after half a year;
- On the 28th day after the third injection of the second group (0-14-194), the neutralizing antibody GMT reached 137.9;
- On the 28th day after the third injection of the third group (0-28-56), the neutralizing antibody GMT reached 49.7;
- On the 28th day after the third injection of group 4 (0-28–208), the neutralizing antibody GMT reached 143.1.
Picture Test result picture
In addition, on July 24, the Department of Immunology of Dalian Center for Disease Control and Prevention and the Key Public Health and Safety Research Office of the Ministry of Education, School of Public Health, Fudan University, Shanghai, uploaded the research article “After Vaccination in Dalian, China” on the preprint platform SSRN. Surveillance for adverse events following immunization with COVID-19 vaccines in Dalian, China (Surveillance for adverse events following immunization with COVID-19 vaccines in Dalian, China) evaluated the safety of mass vaccination of China’s COVID-19 inactivated vaccine in Dalian, Liaoning Province, China.
The research data comes from the Dalian City Immunization Planning Information System and the Chinese National Adverse Events Following Immunization System (CNAEFIS). The researchers analyzed the data of the COVID-19 inactivated vaccination carried out in Dalian from November 27, 2020 to June 8, 2021, and the data reported by Adverse Events Following Immunization (AEFI).
The analysis results showed that during this period, Dalian City had received 7.12 million doses of inactivated COVID-19 vaccine, and a total of 623 cases of AEFI were reported, with an incidence rate of 87.5 per million doses. Among AEFI, 616 cases (98.9%) were non-severe cases, with an incidence rate of 86.5 per million doses; 7 cases were severe cases, with an incidence rate of 1/100 million doses. During the observation period, a total of 6.08 million doses of SINOVAC’s COVID-19 vaccine Kellyf were administered, accounting for 85.4% of all vaccination doses. The incidence of AEFI after being vaccinated with CoronaVac was 81.1 per million doses, the incidence of non-severe AEFIs was 80.3/100 million doses, and the incidence of severe AEFI was 0.8 per million doses.
According to the analysis of the surveillance data of suspected abnormal response to vaccination in China in 2018, the reported incidence of AEFI for vaccination in China that year was 45.89/100,000 doses, and the incidence of severe and non-severe AEFIs were 029/100,000 doses and 45.59/100,000 doses respectively.
In addition, according to a report issued by the Chinese Center for Disease Control and Prevention, as of April 30, 2021, mainland China had received 265 million doses of the COVID-19 vaccine, and the overall AEFI rate was 118.6 doses per 1 million doses, similar to other conventional inactivated vaccines. Common adverse reactions accounted for 82.96% of the total AEFI, and the incidence was 98.4 per million doses.
The incidence of rare adverse reactions was 20.2/100 million doses, most of which were allergic rash and angioedema, which did not lead to serious consequences. Therefore, the incidence of AEFI and the incidence of severe and non-severe AEFIs of CoronaVac are far lower than the reported levels of AEFI of previous vaccines, showing good safety.
SINOVAC published the animal research results of the new coronavirus inactivated vaccine Kellyf and 18-59 year olds in international authoritative journals in May 2020, November 2020, February 2021, and June 2021, respectively. The results of Phase I/II clinical studies in adults, healthy people aged 60 and over, healthy children and adolescents aged 3-17 years, the results of the Phase III clinical trial of Kerlaiford published in Turkey, and mass vaccinations carried out in many countries Practice has confirmed the safety and immunogenicity of CoronaVac, as well as the reliability of large-scale use.
CoronaVac was the first to be approved for emergency use in China in June 2020. On February 5, 2021, the National Medical Products Administration approved its conditional listing in China in accordance with the law, and it was approved in China on May 28 for children aged 3 and above. Emergency use, the World Health Organization included it in the emergency use list on June 1.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



