Analysis of world first nebulized inhalation COVID-19 vaccine Ad5-nCoV
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Analysis of the clinical trial results of the world first nebulized inhalation vaccine Ad5-nCoV
Analysis of world first nebulized inhalation COVID-19 vaccine Ad5-nCoV. On July 28, 2021, the clinical phase I results of the inhaled human type 5 adenovirus vector vaccine Ad5-nCoV developed by the team of Academician Chen Wei of China Academy of Military Medical Sciences and CanSino Biological Co., Ltd. were published in the international top medical journal Published on “The Lancet- Infectious Diseases” [1].

This study is a randomized open phase I clinical trial, carried out in Wuhan Zhongnan Hospital, to evaluate the safety and immunogenicity of Ad5-nCoV in aerosolized inhalation form in healthy adults 18 years and older. A total of 130 subjects were recruited for this clinical trial, and they were randomly divided into 5 experimental groups according to the method of administration and dosage, with 26 subjects in each group:
(1) High-dose inhalation group: Ad5-nCoV (2×10¹⁰viral particles) nebulized inhalation in the form of inhalation on day 0 and 28 respectively;
(2) Low-dose inhalation group: Ad5-nCoV (1×10¹⁰viral particles) nebulized inhalation in the form of inhalation on day 0 and 28 respectively;
(3) Mixed vaccination group (MIX group): received the first intramuscular injection of Ad5-nCoV (5×10¹⁰ virus particles) on day 0 and 28) + the second dose of nebulized Ad5-nCoV (2×10¹⁰viral particles) ;
(4) 1 dose of intramuscular injection group: 1 dose of Ad5-nCoV (5×10¹⁰viral particles) inoculated on day 0;
(5) 2 times the dose intramuscular injection group: vaccination of 1 dose of Ad5-nCoV (10×10¹⁰viral particles) on day 0.
Trial results:
1. Security
Compared with the participants who received the aerosol vaccine, the participants who received intramuscular injection had more adverse events. When the first dose of Ad5-nCoV was vaccinated, the aerosol inhalation method showed significantly better safety than intramuscular injection, and the incidence of adverse reactions (25%) is significantly lower than intramuscular injection (63%) (χ²=17.89, df=1.00, p<0.0001).
Within 7 days after the first dose of the vaccine, 62 (48%) of the 130 participants reported at least one adverse event, and no serious adverse reactions occurred. The most common systemic adverse events were fever (≥37.3°C; 35[27%] of 130 participants), headache (20[15%]) and fatigue (17[13%]).
There was no significant difference in adverse respiratory events, such as cough, oropharyngeal pain, and swelling, between participants who received aerosol and participants who received intramuscular injections (p=0.68).
The safety observation of two doses of aerosol inhalation of Ad5-nCoV for 28 days confirmed that the two doses of aerosol Ad5-nCoV were well tolerated and did not cause any serious adverse events related to the vaccine.
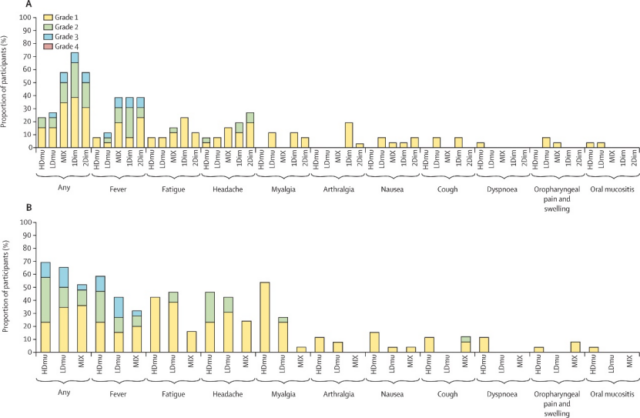
Figure 1 Adverse events observed after Ad5-nCoV vaccination. (A) Adverse events reported within 7 days after the first dose of vaccination; (B) Adverse events reported within 7 days after the second dose of vaccination. 2 doses of high-dose aerosol inhalation-HDmu, 2 doses of low-dose aerosol inhalation-LDmu, mixed vaccination-MIX, 1 dose of intramuscular injection -1 Dim, 2 times dose intramuscular injection of 2 Dim
2. Immunogenicity
Measure serum IgG and IgA before and after vaccination, and the titers of neutralizing antibodies; CD4 and CD8 T cells and interferon-γ (IFN-γ) are used to reflect cellular immunity.
1) One dose of aerosol Ad5-nCoV, equivalent to one-fifth of the muscle dose, can induce a strong cellular response, and two doses of aerosol Ad5-nCoV can also produce a similar response.
2) Re-immunization with inhaled Ad5-nCoV after intramuscular injection of Ad5-nCoV can significantly increase the level of antibody and cellular immunity.
3) The humoral immunity level was compared among the 5 experimental groups. Whether it was neutralizing antibody level or IgG or IgA, the level of antibody stimulated by intramuscular injection + inhalation mixed vaccination group was significantly higher than that of other groups. Comparing the cellular immune responses of each group, a significant increase in cellular immunity can also be observed in the mixed vaccination group after the second dose of inhalation vaccine.
4) Vaccination through aerosol inhalation can provide additional mucosal immune protection.
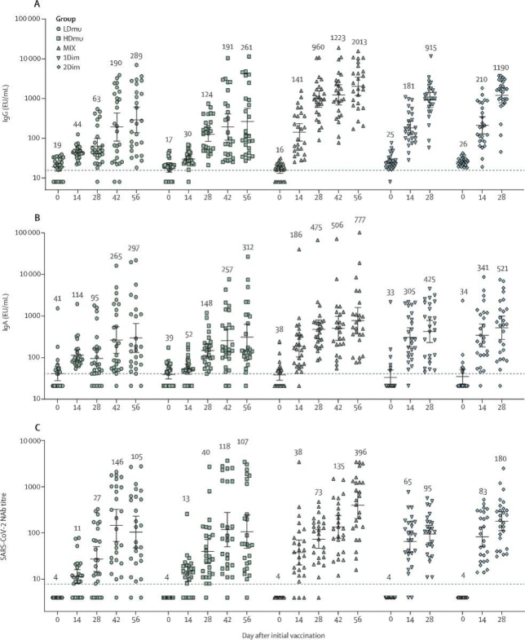
Figure 2 The levels of (A) serum IgG antibody, (B) IgA antibody level, and (C) neutralizing antibody level after inoculation of Ad5-nCoV vaccine by different routes.
Discussion of Analysis of world first nebulized inhalation COVID-19 vaccine Ad5-nCoV
This study is the world’s first publicly published results of a clinical study on mucosal immunity of the COVID-19 vaccine. It provides valuable experience for the subsequent non-injection-administered COVID-19 vaccines and other vaccines under development for adenovirus vectors. As of June 3, 2021, according to statistics from WHO and ClinicalTrials.gov, there are 102 new coronavirus vaccines under development in the world that have entered the clinical stage. The new coronavirus vaccines are vaccinated through non-injection methods (including oral, nasal spray, atomized inhalation, etc.) A total of 14 [1].
Previously, the injection form of Ad5-nCoV has shown good safety and immunogenicity in people aged 18 and over [2,3]. The interim analysis data of its international multi-center phase III clinical trial showed that Ad5-nCoV was 14 days after vaccination. The protection rate for severe cases is 95%, and the protection rate for all symptomatic cases is 68.83% [4]. The clinical study of inhaled Ad5-nCoV published this time shows that the human type 5 adenovirus vector COVID-19 vaccine Ad5-nCoV vaccine of the same formulation composition can be vaccinated by aerosol inhalation administration route other than intramuscular injection, and shows good Safety, tolerability and immunogenicity.
The SARS-CoV-2 neutralizing antibody response triggered by two doses of Ad5-nCoV in aerosol is similar to that of one dose of this vaccine intramuscularly. Nebulized inhalation booster immunization on the 28th day after intramuscular injection of Ad5-nCoV can induce a strong IgG and neutralizing antibody response. The inhaled vaccine immunization method is economical and effective, and further evaluation of this immunization method should be carried out.
Compared with the commonly used intramuscular injection formulations, aerosol inhalation formulations do not require injections, can eliminate local adverse reactions, and have better safety and convenience. In addition, the administration method of nebulized inhalation can stimulate the mucosal immune response and provide two additional layers of protection in the respiratory mucosal tissues: secreted IgA antibodies, memory B cells, and memory T cells. To establish mucosal immunity in the respiratory mucosa, memory B cells and T cells distributed in the respiratory mucosa encounter pathogens earlier than systemic memory cells, which can inhibit virus replication faster and reduce virus transmission [5]. The new coronavirus usually enters the human body from the respiratory tract, and the establishment of a mucosal immune barrier on the first line of defense of the virus is of great significance to preventing infection and blocking the spread of the virus.
The goal of vaccination is to obtain lasting protective immunity. The vaccination strategies of different immunization routes are conducive to improving the protective efficacy of the vaccine. First, intramuscular injection of the vaccine induces a long-lasting IgG antibody response throughout the body to produce long-lasting memory cells; then, non-injection vaccination is used to enhance the recruitment of memory cells to the nasal cavity, induce mucosal immunity, and finally obtain long-lasting protection. Immunization may be one of the ideal vaccination strategies for obtaining persistent immunity [5]. In addition, different vaccination routes can produce different antibody compositions; compared with intramuscular vaccination, aerosol vaccination may trigger a higher ratio of neutralizing antibodies in total antibodies.
This research has important implications for the prevention and control of the COVID-19 epidemic in the future. Especially in the current frequent mutations of the COVID-19 virus, whether it is an individual who has been vaccinated with the COVID-19 vaccine or a population who is about to be vaccinated with the COVID-19 vaccine, they are facing the question of whether the vaccine’s effectiveness should be strengthened.
Scholars at home and abroad are discussing whether to increase the number of vaccination doses. At present, more and more evidence supports the sequential immunization of the COVID-19 vaccine produced by different research and development routes, which can significantly enhance the immune efficacy of the vaccine.
According to this idea, based on the current COVID-19 vaccination procedure, if the inhaled human type 5 adenovirus vector vaccine Ad5-nCoV is used as a booster, it has the following advantages:
- First, it is convenient to implement and takes advantage of the inhaled vaccination route;
- It can take advantage of the sequential immunization method to enhance cellular immunity and humoral immunity;
- third, because the dose of the inhaled human type 5 adenovirus vector vaccine is only one-fifth of the injected dose, it can save a lot of vaccine consumption for more extensive use.
Population vaccination, which highlights its significance when the global COVID-19 vaccine is in short supply and a vaccine is hard to find.
In summary, the role of the inhaled human type 5 adenovirus vector vaccine Ad5-nCoV in the future prevention and control of the COVID-19 epidemic is worth looking forward to; this vaccine is also an option to strengthen the COVID-19 vaccination program, and it is also worthy of active exploration.
The study also has certain limitations. The main reason is that the sample size is small, and the dose-dependent immune response has not been observed in the participants receiving aerosol vaccines. In addition, the relationship and role of inhalation vaccination and mucosal secretion of IgA antibodies are worthy of more in-depth discussion. Since this is only a phase 1 clinical trial, we expect that there will be more data in the future phase 2 and phase 3 clinical trials to enrich people’s understanding of the inhaled human adenovirus type 5 vector vaccine.
Analysis of world first nebulized inhalation COVID-19 vaccine Ad5-nCoV
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



