Humoral immune response of different COVID-19 vaccines to mutantions
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Cell Research: Humoral immune response of different COVID-19 vaccines to mutantions
Humoral immune response of different COVID-19 vaccines to mutantions. With the continuous spread of the new coronavirus (SARS-CoV-2), its mutant strains such as B.1.1.7 (British strain), B.1.351 (South African strain), B.1.617 (Indian strain), etc. began to spread all over the world appear.
The COVID-19 mutant strain can cause immune escape through mutations in the receptor binding domain (RBD) and N-terminal domain (NTD) of the spike protein (S protein). These mutations respond to the humoral immune response of COVID-19 patients and vaccinators There is still a lack of systematic research on the impact of antibody recognition and antibody recognition.
On May 21, 2021, Peking University Biomedical Frontier Center (BIOPIC), Beijing Future Genetic Diagnosis Advanced Innovation Center (ICG) Xie Xiaoliang team, etc. published in Cell Research the title: Humanal immune response to circulating SARS-CoV-2 A research paper on variants elicited by inactivated and RBD-subunit vaccines.
The research team used a variety of technical methods such as high-throughput single-cell sequencing, cryo-electron microscopy, virus neutralization experiments and other technical methods to analyze the new coronavirus inactivated vaccine (Kexing) and RBD subunit recombinant protein vaccine (Zhifei) vaccinators and the recovery of new coronavirus infections The response of the patient’s plasma and induced neutralizing antibodies to the currently circulating COVID-19 mutant strains (such as the South African strain).

The research team used high-throughput single-cell sequencing to screen and express 831 anti-spike protein antibodies from the peripheral blood memory B cells of the two types of vaccine inoculators and recoverers, and screened 86 wild-type antibodies against the new coronavirus. Type of antibody with high neutralizing activity.
Pseudovirus neutralization test experiments show that more than half of the anti-RBD neutralizing antibodies have a significant decrease in the neutralizing activity of the K417N / E484K / N501Y mutation combination. The structure of high-resolution cryo-electron microscopy shows that the cause of the significant decrease in neutralizing antibody activity is the charge reversal caused by the E484K mutation.
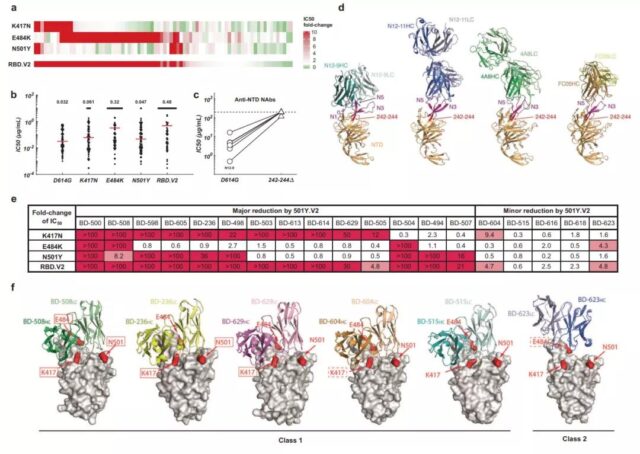
Immune response of anti-RBD and anti-NTD neutralizing antibodies to the N501Y mutant strain of new coronavirus
Nevertheless, due to the diversity of the epitope distribution of anti-RBD neutralizing antibodies, they have shown a high degree of diversity when dealing with RBD mutations such as K417N / E484K / N501Y. In contrast, the deletion of position 242-244 (242-244Δ) on the NTD carried by the South African strain will change the protein conformation of the NTD antigen hot spot, thereby eliminating most of the neutralizing activity of the anti-NTD antibody, which means that the anti-NTD antibody The diversity of NTD neutralizing antibodies is far less than that of anti-RBD neutralizing antibodies.
In order to further confirm the diversity of anti-RBD antibodies, the research team analyzed the neutralizing ability of 18 VH3-53/VH3-66 genotype antibodies against the South African strain. VH3-53/VH3-66 type antibodies are antibodies that appear in the body of different infected persons. These antibodies have the same epitope and structural height on the RBD. Nevertheless, the reactivity of VH3-53/VH3-66 antibodies to mutant strains is still different. For example, a small number of VH3-53/VH3-66 are not affected by mutant strains, and most VH3-53/VH3-66 antibodies are against K417N. The / E484K / N501Y mutant strain failed. Structural analysis proved that this part of the failed antibody was mainly caused by the K417N mutation reducing the polar interaction between the complementarity determining region of the antibody and the RBD.
Similar to the results of the neutralizing antibody experiment, the plasma of the recovered and inactivated vaccine recipients showed a trend of decreasing neutralizing activity against the South African mutant. The results of the neutralization experiments of pseudoviruses and true viruses showed that compared with the common epidemic strains, the neutralizing activity of the plasma from patients who recovered from COVID-19 infection and inactivated vaccines against the South African strain decreased by about 4 times. The reason is mainly caused by E484K/N501Y and 242-244Δ mutations, and the degree of weakening of plasma neutralization activity by mutations in these two regions can be superimposed. The plasma neutralization activity of the RBD subunit recombinant protein vaccine was only reduced by about 2 times in the true virus and the pseudovirus, indicating that the plasma of the RBD subunit recombinant protein vaccine inoculated to the South African strain is better than that of the recovered plasma. The main reason is that the anti-RBD neutralizing antibodies induced by it have a rich diversity and are not affected by NTD mutations.
This study also found that compared with the standard three doses of RBD recombinant protein subunit vaccine (day 0/30/60), the interval between the third and second doses of recombinant protein vaccine was extended (the first 0 days/30 days/140 days) will produce antibodies with stronger neutralizing activity and stronger tolerance to South African mutant strains. These results indicate that if COVID-19 mutant strain is pandemic in the future, especially for virus strains that can change the conformational mutation of NTD antigen hotspot protein, injection of a third dose of RBD recombinant protein vaccine to boost immunization may be an optimized plan.
(source:internet, reference only)
Disclaimer of medicaltrend.org



