Cell: The first time to clarify the effect of new coronavirus antibody ADE
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Cell: The first time to clarify the effect of new coronavirus antibody ADE
Cell: The first time to clarify the effect of new coronavirus antibody ADE. The picture vaccine is an important measure to control the COVID-19 epidemic. At the same time, neutralizing antibodies can also be used as drugs to prevent and treat infections.
Many studies have shown that SARS-CoV-2 neutralizing antibodies can effectively block viral infections in primates and mouse models. Neutralizing antibodies targeting the RBD domain of the new coronavirus are more effective, while antibodies targeting NTD neutralize The ability is weak.
There has always been a controversy regarding the clinical treatment of antibodies, that is, antibody-dependent enhancement (ADE) infection. In SARS-CoV and other viral infections and vaccines, it has been found that the virus can bind to target cells through cell surface IgG antibody Fc receptors and complement receptors to promote infection.
However, the current ADE effect on the new coronavirus has not been experimentally confirmed. A team has identified the binding sites of infection-enhancing antibodies. Recently, Duke University in the United States and the National Institutes of Health, Harvard University, and Columbia University have published CELL Published a research paper on the first time in vitro and in vivo experiments to clarify the complex impact of the antibody ADE effect in the new coronavirus infection.

The researchers isolated monoclonal antibodies derived from plasma cells or memory B cells from individuals infected with SARS-CoV-2 at different time points, and isolated antibodies that were active against both the new coronavirus and SARS from individuals who had been infected with SARS. Using high-throughput screening, the researchers obtained 187 strains of antibodies with high response levels, of which 44 strains of RBD neutralizing antibodies showed neutralizing efficacy against pseudoviruses and replicable viruses, and 10 strains of NTD neutralizing antibodies had an IC50 of 39ng/ mL.
In addition, five non-neutralizing NTD antibodies promoted the infection of new coronavirus in 293T and Vero cell models. Since the cell line used does not express Fc receptors, NTD antibodies do not rely on FcγR to exert ADE effects, and mediate antibody-dependent enhancement. It is due to ACE2 expression. In order to analyze the enhancement of Fc receptor-dependent infection, the effect of antibodies on virus infection was tested in the TZM-bl cell line. The results showed that it can indeed promote SARS-CoV-2 infection. RBD neutralizing antibodies can both neutralize 293T cells. Efficacy can also enhance infection in the TZM-bl cell line.
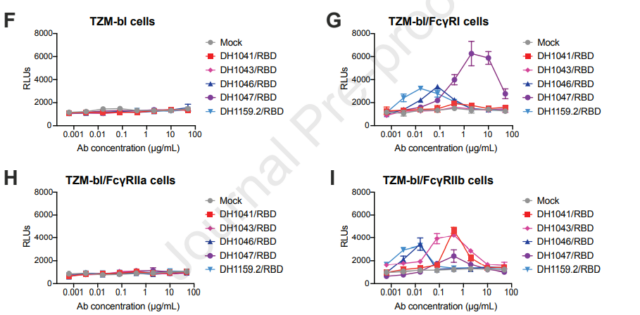
ADE effect
In order to understand the mechanism by which these antibodies enhance infection, the researchers analyzed the phenotype and binding mode, and the results showed that the affinity and neutralizing activity of infection-enhancing antibodies or non-enhancing RBD antibodies are similar. There was no difference between the two types of antibodies in the electron microscope counterstaining observation. The researchers further analyzed that the affinity of the Fab fragment of the NTD neutralizing antibody to the extracellular end of the S protein was up to 19 nM, while the affinity of the infection-enhancing antibody was only 294 nM. The infection-enhancing NTD antibody Fab region and the S protein binding site are close to the virus envelope, while the neutralizing antibody is the opposite, and the epitope is mainly 4A8.
Neutralizing antibodies and infection-enhancing antibodies
The researchers used surface plasmon resonance technology to analyze the competitiveness of infection-enhancing antibodies and non-enhancing antibodies, and found that there are two types of binding epitopes in both RBD and NTD. Antibodies targeting the same epitope compete with each other, and antibodies against different epitopes are different. At the same time, RBD antibody and NTD antibody do not affect each other and can bind to S protein trimer at the same time. In particular, the researchers analyzed the enhancing antibody DH1052 and the neutralizing antibody DH1041, and speculated that the infection result depends on the higher concentration of the antibody. Only when the concentration of neutralizing antibody is less than 10ng/mL, that is, less than 1/1000 of the enhancing antibody, will it cause antibody-dependent enhancement, which does not occur in natural infections.
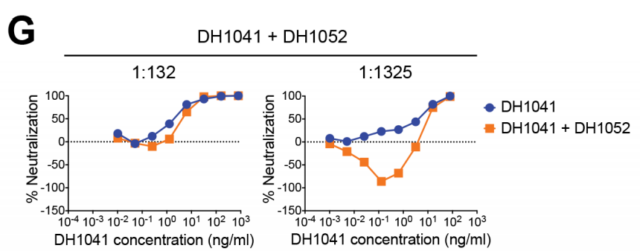
Enhancing the competition between antibodies and neutralizing antibodies
The researchers used cryo-electron microscopy to observe the interaction between different antibodies and S protein. The results showed that the extracellular end of S protein showed a heterogeneous conformation of S-2P, and all S-2P trimers bound the Fab of the three antibodies. The epitopes of RBD neutralizing antibodies are mainly located in the RDM region, while other antibodies bind to some residues outside the RBM. The NTD neutralizing antibody binds to the host cell membrane, while the enhancing antibody binds to the NTD.
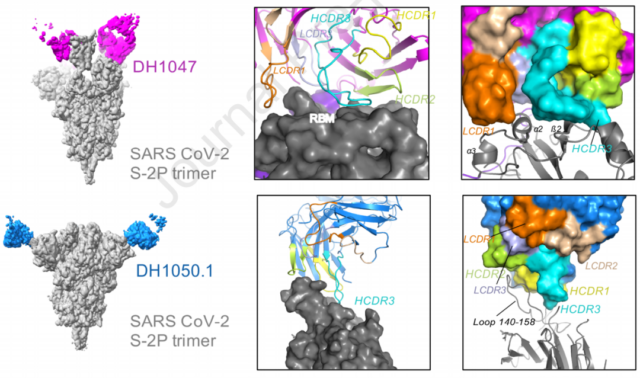
Cryo-electron microscopy analysis of two antibody structures
The researchers then tested the effect of the NTD infection-enhancing antibody on the COVID-19 mouse model, and found that the severity of the disease in the mice was reduced after treatment with the antibody, and the viral replication load decreased. This may be due to the binding of antibodies to FcR receptors to promote innate immunity. The effect of NTD antibody was further tested on the rhesus monkey model. The neutralizing antibody obviously neutralized the new coronavirus, while the enhanced antibody could not block the virus. However, several rhesus monkeys after receiving the enhanced antibody treatment developed lung inflammation, even Infiltration of vasculitis cells and up-regulation of cytokines. Receiving neutralizing antibodies and enhancing antibodies both reduced the viral load. At higher doses of enhanced antibodies, virus replication was significantly reduced, and no immunopathological features appeared. The situation of RBD antibodies is basically similar. Both neutralizing antibodies and enhancing antibodies can effectively block virus replication in mouse and primate models, and only a few individuals show higher inflammatory infiltration.
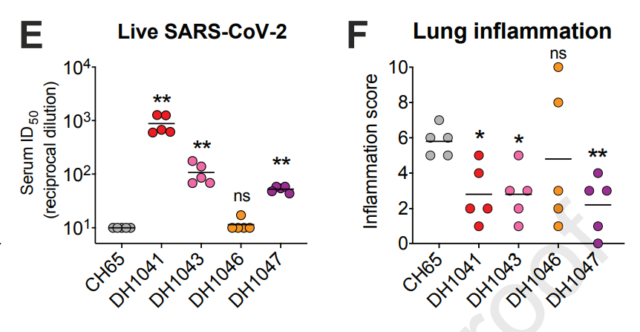 In vivo antibody efficacy
In vivo antibody efficacy
The study shows that infection-enhancing antibodies can promote new coronavirus infection in vitro, but will not cause ADE effects in vivo, which provides strong support for neutralizing antibody applications and vaccine design.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



