Does NSCLC immunotherapy require concurrent radiotherapy and chemotherapy?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Does NSCLC immunotherapy require concurrent radiotherapy and chemotherapy?
Does NSCLC immunotherapy require concurrent radiotherapy and chemotherapy? Immunotherapy + radiotherapy and chemotherapy to conquer locally advanced NSCLC.
The 2021 American Society of Clinical Oncology (ASCO) annual meeting will be held online from June 4th to 8th. As one of the largest and most popular events in the oncology field, it will show scholars from all walks of life the latest cutting-edge progress in the field of oncology every year. T
This article will interpret the important research on immunity + radiotherapy and chemotherapy of advanced non-small cell lung cancer from different perspectives and convey cutting-edge trends.
PACIFIC trial: 5-year follow-up results of unresectable stage III NSCLC patients who received chemotherapy and durvalumab (duvalizumab/PD-L1)
The treatment mode for stage III insignificant non-small cell lung cancer before 2017 was concurrent radiotherapy and chemotherapy, and after 2017, the treatment mode became concurrent radiotherapy and chemotherapy + immunotherapy. In this context, more and more clinical trials involving immunotherapy have been launched. The PACIFIC trial is a phase III double-blind randomized controlled trial. The enrolled 713 patients were unresectable phase III NSCLC patients, all ages greater than or equal to 18 years, and WHO PS score of 1 or 0, all completed At least two cycles of radiotherapy and chemotherapy. The treatment plan of the experimental group was duvalizumab, 10mg/kg, Q2W, lasting one year; the treatment plan of the control group was Placebo, 10mg/kg, Q2W, lasting one year.
1. The 5-year survival rate of patients in the experimental group was 42.9%, and OS and PFS continued to benefit
The 5-year survival rate of the experimental group is close to 10% higher than that of the control group, and the 5-year survival rate of 42.9% has even reached the survival rate of patients with early stage lung cancer undergoing surgical resection.

Radiotherapy and chemotherapy + immunosuppressive treatment: OS and PFS continue to benefit
2. Patients with EGFR+ and PD-L1<25% have little benefit from OS
In the experimental group, EGFR+ patients, PFS and OS did not benefit significantly. Therefore, whether these patients are suitable for radiotherapy and chemotherapy + immune checkpoint inhibitor treatment mode still needs to be further explored.
For EGFR+ patients, the current use of third-generation TKI inhibition can achieve a better curative effect. PD-L1<25% of patients benefited from PFS, but OS did not.
Therefore, if we want to adopt the mode of radiotherapy and chemotherapy + immune checkpoint inhibitor therapy for such patients, we may need to consider the detection of PD-L1 expression.
3. Elderly patients can tolerate radiotherapy and chemotherapy + immune checkpoint inhibitor therapy, but PFS has no obvious benefit.
Based on the background that the PACIFIC study has brought significant OS and PFS benefits to patients with stage III NSCLC, another study has explored whether elderly patients (70-90 years old) are suitable for this treatment mode. The results showed that although the mode of radiotherapy and chemotherapy + duvalizumab did not benefit elderly patients from PFS (P>0.05), there were no obvious serious immune side effects.
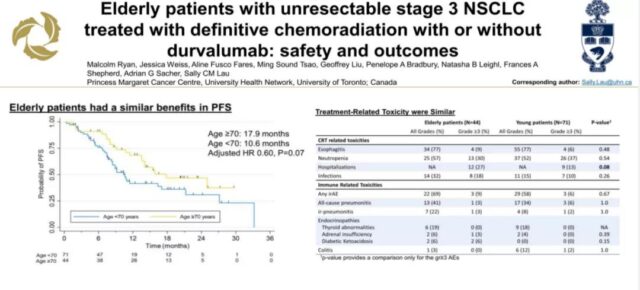
Elderly patients have no significant PFS benefit
KEYNOTE-799 trial: Phase II clinical trial of Pembrolizumab + platinum-based chemotherapy and radiotherapy for patients with unresectable stage III NSCLC
The PACIFIC study found that patients who received duvalizumab early within 14 days of concurrent chemoradiation and chemotherapy could obtain better OS compared to starting duvalizumab treatment 14 days after concurrent chemoradiation. Such results suggest that our early application of immunotherapy may benefit patients more.
Based on this background, the KEYNOTE-799 trial was launched. This trial also included patients with unresectable stage III NSCLC, all ages greater than or equal to 18 years, ECOG score of 1 or 0, and no immunotherapy within 7 days. Patients were divided into groups A and B. Patients in group A were treated with pembrolizumab + paclitaxel + carboplatin in the first cycle, and pembrolizumab + paclitaxel + carboplatin + radiotherapy were used in the second and third cycles.
Pembrolizumab single-agent maintenance treatment was used in cycles 4 to 17. Patients in group B were treated with pembrolizumab + pemetrexed + cisplatin in cycle 1, pemetrexed + cisplatin + radiotherapy were used in cycles 2 and 3, and pembrolizumab was used in cycles 4 to 17 Single-agent maintenance treatment.
1. The effect of immune advancement combined with concurrent radiotherapy and chemotherapy is beginning to show
The ORR value of group A and group B reached more than 70%, and the ORR value was not affected by the level of PD-L1 expression. It shows that the early application of immune checkpoint inhibitors has a certain effect. Due to insufficient follow-up time, the results of OS and PFS have not been published.
2. The occurrence of interstitial pneumonia is in line with expectations and still needs attention
The incidence of immune-related pneumonia in the two groups above grade 3 was less than 10%, which was within the test expectations, but higher than the PACIFIC study results. Therefore, this treatment model needs more evidence to support.
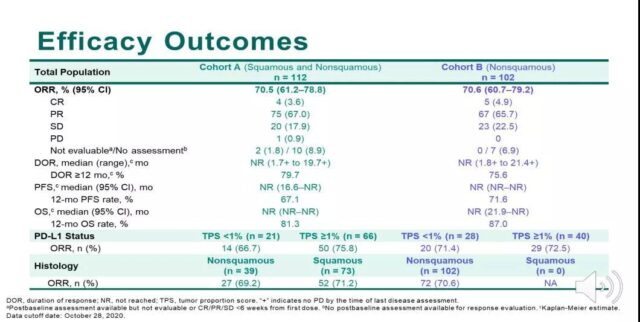
Immunization advancement combined with concurrent radiotherapy and chemotherapy has a higher ORR value and has a certain effect
AFT-16: Phase II clinical trial of neoadjuvant and adjuvant atezolizumab treatment and radiotherapy and chemotherapy (CRT) for phase III non-small cell lung cancer (NSCLC)
In the KEYNOTE-799 study, early use of immunotherapy has achieved initial results, but what about the ultra-early use of immunotherapy? The AFT-16 study is to use immunotherapy as a neoadjuvant treatment to explore such problems. AFT-16 also included patients with stage III non-small cell lung cancer who had no previous autoimmune diseases. All patients have completed 2 cycles of atilizumab treatment, after which the patients were divided into 2 groups, the experimental group was treated with atilizumab (2 cycles) + standard radiotherapy and chemotherapy + adjuvant atilizumab Anti-treatment; and the control group directly used standard radiotherapy and chemotherapy.
1. The immune checkpoint inhibitor atelizumab is safe for patients before and after radiotherapy and chemotherapy, and the effect is obvious.
2. The median PFS reached 23.7 months, and the OS reached 18 months in 84% of patients.
Compared with the median PFS of about 17 months in PACIFIC, the ultra-early application of immunotherapy can reach 23.7 months. It shows that the ultra-early application of immunotherapy has certain advantages over the mode of concurrent radiotherapy and chemotherapy + immunotherapy. However, the sample size of the study is relatively small, and more evidence is needed to support it.
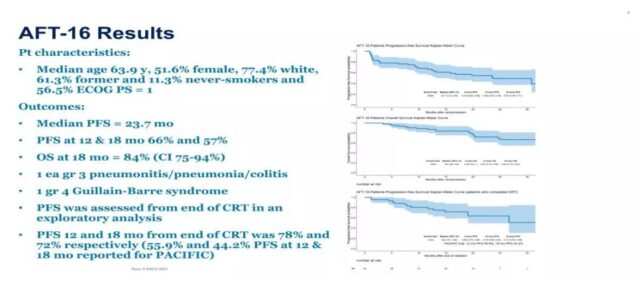
Ultra-early immunotherapy has obvious advantages in PFS and drug safety
Sum up
1. Simultaneous radiotherapy and chemotherapy are the cornerstones
Although the emergence of immunotherapy has brought new treatment modes, no matter which treatment mode, they are inseparable from concurrent chemoradiotherapy, so concurrent chemoradiotherapy is still the cornerstone of the treatment of locally advanced NSCLC.
2. The form of immune intervention still needs to be explored
The treatment model of immune intervention, whether it is ultra-early, synchronized with radiotherapy and chemotherapy, or single-agent maintenance treatment after concurrent radiotherapy and chemotherapy, requires a large amount of clinical trial evidence to support.
3. What is the optimal model for positive driver gene?
For patients with positive driver genes, immunotherapy + concurrent radiotherapy and chemotherapy have no obvious benefits, and this type of patients accounts for a large proportion, so further research on better treatment options is needed.
4. What is the dose and schedule of concurrent radiotherapy and chemotherapy in the era of immune intervention?
After immune intervention, whether the dose and schedule of traditional concurrent radiotherapy and chemotherapy should be adjusted accordingly, and how to adjust it is also a question that needs to be further explored in the future.
Does NSCLC immunotherapy require concurrent radiotherapy and chemotherapy?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



