Why are the results of clinical trials of COVID-19 drugs inconsistent?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Why are the results of clinical trials of COVID-19 drugs inconsistent?
Why are the results of clinical trials of COVID-19 drugs inconsistent? A new modeling study shows that significant differences in viral dynamics between different patients may be the reason for the inconsistent clinical trial results of COVID-19 drugs.
A new modeling study shows that significant differences in viral dynamics between different patients may be the cause of inconsistent clinical trial results of COVID-19 drugs.
Shoya Iwanami of Nagoya University in Japan and Keisuke Ejima of Indiana University in the United States said that if clinical trials recruit participants who have just developed symptoms, the number of participants needed to detect the effects of antiviral drugs can be greatly reduced. They published these results in the open-access journal PLOS Medicine.
An effective COVID-19 antiviral drug will have a major impact on global health. However, global clinical trials testing drug candidates have produced inconsistent results, which may be due to flaws in the design of the trials.
To solve this problem, Iwanami and colleagues first used the kinetic model of SARS-CoV-2 infection in humans. They combined the model with clinical data to examine how the viral load (that is, the amount of virus in the human throat) changes over time, and found significant differences in the rate of viral decline between different patients. These differences may have led to inconsistent results reported in non-randomized clinical trials.
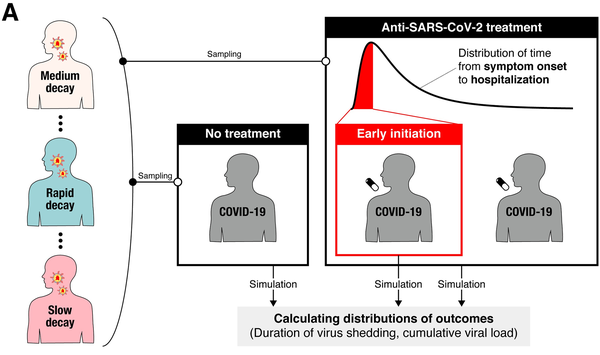
A randomized controlled clinical trial of simulated anti-SARS-CoV-2 (picture from original)
Next, the researchers simulated the possible results of a randomized clinical trial of a COVID-19 drug that successfully blocked virus replication. They found that even if a drug reduces virus replication by 95%, related randomized clinical trials require the recruitment of more than 13,000 people to participate in the drug trial, plus the same number of controls to detect significant differences in viral load. . In most cases, this number is quite large and unreasonable.
However, when the researchers changed the simulated randomized clinical trial to allow participants to receive treatment one day after the onset of symptoms, they found that each group only needed less than 600 participants. This shows that randomized clinical trials of COVID-19 drugs can be improved in two ways: one is to recruit participants who have just developed symptoms as soon as possible, and the other is to set recruitment criteria based on the time after symptoms appear.
The researchers pointed out that future studies can use more detailed SARS-CoV-2 kinetic models to more reliably calculate the number of participants required for randomized clinical trials to produce consistent results.
Dr. Shingo Iwami of Nagoya University added: “We have found that if patients are recruited into clinical trials regardless of whether symptoms appear, the number should exceed 10,000, which is a bit unreasonable. This is because many patients are being recruited. The recruitment was too late to see the effect of antiviral treatment.”
“Therefore, we recommend recruiting only those who have just developed symptoms. If we only recruit patients within two days of the onset of symptoms, then only 500 patients need to be recruited. The method we have developed can also be applied to other types of drugs and different types of drugs. In infectious diseases. We hope to develop an online platform that supports the design of clinical trials,” he said.
###
Detection of significant antiviral drug effects on COVID-19 with reasonable sample sizes in randomized controlled trials: A modeling study
Published: July 6, 2021
https://doi.org/10.1371/journal.pmed.1003660
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



