Moderna COVID-19 vaccine Safe and Effective in Phase 2-3 trials of age 12-17
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Moderna mRNA COVID-19 vaccine is safe and effective in phase 2-3 clinical trials of 12-17 year olds
- Israel new drug for COVID-19: EXO-CD24 can reduce deaths by 50%
- COVID-19 vaccines for children under 12 will be available soon
- Breakthrough infection of Delta: No difference from regular COVID-19 cases
- French research: ADE occurred in Delta variant and many doubts on it
- The viral load of Delta variant is 1260 times the original COVID-19 strain
Moderna COVID-19 vaccine Safe and Effective in Phase 2-3 trials of age 12-17. The global pandemic of the COVID-19 virus has caused more than 200 million infections and more than 4 million deaths worldwide. Statistics from April 1, 2021 to June 11, 2021 show that the incidence of COVID-19 pneumonia among adolescents between the ages of 12 and 17 is about 9‰.
Although the symptoms of children and adolescents infected with the COVID-19 are usually milder than those of adults, it can also cause severe illness. About one-third of adolescent patients hospitalized with the COVID-19 need to enter the ICU. Therefore, it is necessary to determine the protective effect of the COVID-19 vaccine on young people as soon as possible.
The COVID-19 mRNA vaccine (mRNA-1273) developed by Moderna has confirmed its safety and effectiveness in a number of large-scale clinical trials. It has also received emergency approval from the FDA in December 2020 and received large-scale vaccination. However, Previous clinical trials were conducted in adults. The safety, immunogenicity and protection of the vaccine for children under 18 years of age are still unclear.
On August 11, 2021, Moderna researchers published a Phase 2/3 clinical trial paper titled Evaluation of mRNA-1273 SARS-CoV-2 Vaccine in Adolescents in the New England Journal of Medicine (NEJM).
This Phase 2 randomized controlled clinical trial shows that the mRNA-1273 vaccine is safe and effective for adolescents aged 12-17.

In this ongoing Phase 2/3 placebo-controlled clinical trial, the research team randomly assigned healthy adolescents (12 to 17 years old) at a 2:1 ratio to receive two doses of mRNA-1273 vaccine (100 μg each time) or placebo Injections were administered 28 days apart.
The main goal is to evaluate the safety of mRNA-1273 in adolescents in a phase 3 trial, as well as adolescent immune response and non-inferiority compared with young adults (18 to 25 years of age). Secondary goals include the efficacy of mRNA-1273 in preventing COVID-19 infections or asymptomatic infections.
A total of 3732 participants aged 12-17 years were randomly assigned to receive the mRNA-1273 vaccine (2489 participants) or placebo (1243 participants).
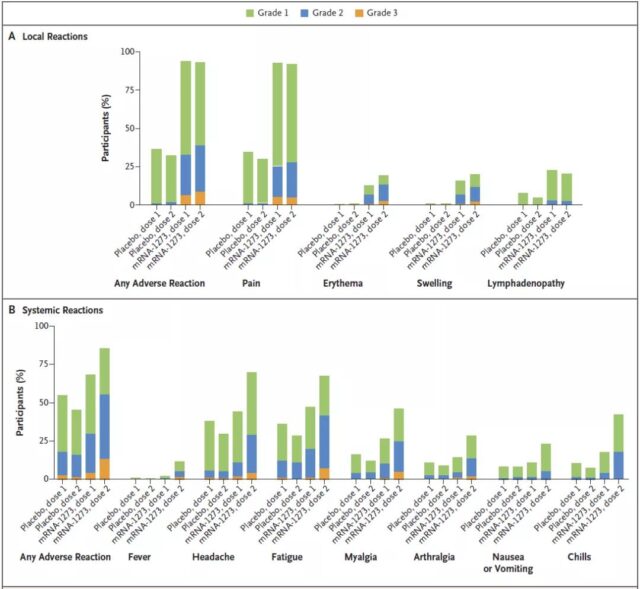
In the mRNA-1273 group, the most common adverse reactions after the first or second injection were pain at the injection site (93.1% and 92.4%, respectively), headache (44.6% and 70.2%, respectively) and fatigue (respectively 47.9% and 67.8%); in the placebo group, the most common adverse reactions after the first or second injection were pain at the injection site (34.8% or 30.3%, respectively), headache (38.5% and 30.2%, respectively) ) And fatigue (36.6% and 28.9%, respectively). None of them had serious adverse reactions.
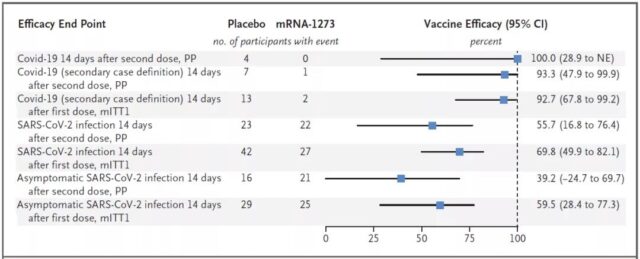
The ratio of the geometric mean neutralizing antibody titer of adolescents (12-17 years old) after receiving two doses of mRNA-1273 vaccine to young adults (18-25 years old) was 1.08, and the absolute difference in serological response was 0.2% , Meet the non-inferiority standard. In other words, the immune response of the mRNA-1273 vaccine in adolescents (12-17 years old) is not lower than that of young adults (18 to 25 years old).

In addition, no one was infected with the COVID-19 14 days after the second dose of the mRNA-1273 vaccine, while 4 people in the placebo group were infected with COVID-19.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



