Should you do antibody test after receiving COVID-19 vaccine?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Should you do antibody test after receiving COVID-19 vaccine? How do you know if the vaccine works?
Should you do antibody test after receiving COVID-19 vaccine? As more and more people are vaccinated with the COVID-19 vaccine, a question has begun to be raised repeatedly: Has the vaccine I vaccinated has worked?
Generally speaking, to evaluate whether a vaccine is effective requires sufficient phase III clinical trial data to support, which in turn means that R&D personnel need to invest a lot of effort to organize trials of tens of thousands of people in areas where the epidemic is prevalent, and they will go through a long period of time The follow-up and waiting for the final answer.
However, a new study may change this situation.
On June 24, the world’s first research on the immunological protection related indicators of the COVID-19 vaccine was launched on the preprint platform medRxiv, which provided an important basis for setting the immunological alternative endpoint of the COVID-19 vaccine in the future, and also answered two crucial points. problem:
After vaccinated with the COVID-19 vaccine, how can I confirm whether I am effectively protected?
How can the new COVID-19 vaccine be confirmed as quickly as possible without a phase III trial?

medRxiv screenshot
What does the new study say?
This study, which has just been launched, is from the University of Oxford, UK. The research team determined the relationship between the level of neutralizing antibodies and the protection of the vaccine by measuring the antibody levels of more than 1,500 vaccinators, and then continuously monitoring the subsequent infections of these people.
The subjects were part of the Oxford/AstraZeneca COVID-19 vaccine COV002 phase III trial in the UK. 4369 people in the experimental group received two doses of AstraZeneca chimpanzee adenovirus vaccine, and 4194 people in the placebo group received MenACWY meningococcal vaccine. These subjects took blood for antibody testing 28 days after the second injection, and then performed nucleic acid testing once a week for 6 months.
Nucleic acid-positive subjects were divided into symptomatic infections (at least one of fever, cough, fatigue, loss of smell and taste, and dyspnea), non-main symptomatic infections (symptomatic but not the above five) and no symptoms according to the symptoms of the disease. Symptoms of infection. More than 60% of the subjects were medical staff.
The results show that the Oxford/AstraZeneca COVID-19 vaccine has an effective rate of 73.1% against common symptomatic infections and 15.1% against asymptomatic infections within 6 months. These data are similar to those of AstraZeneca’s other phase III clinical trials. The results are basically consistent.
The most important purpose of this study is to explore the relationship between neutralizing antibodies in peripheral blood and effective rates.
The study measured the antibody levels of more than 1,500 vaccinators, and then separately counted their infection rates, calculated the infection risk and vaccine protection efficacy of different antibody levels, and the results showed that they were still infected after vaccination (that is, a breakthrough in the vaccine). Infected people are indeed the lower part of the vaccinated group.
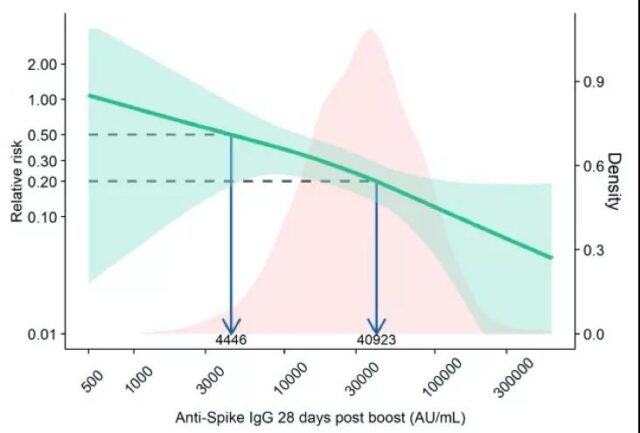
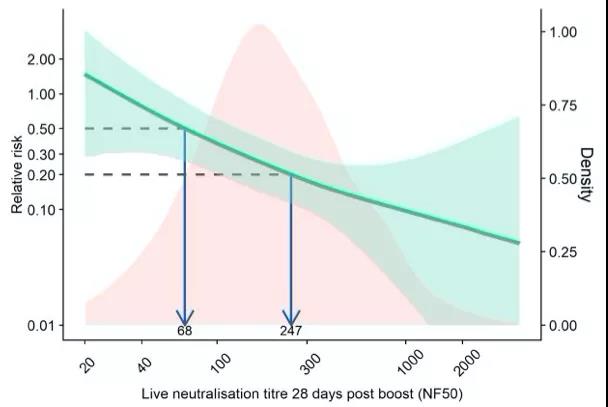
Correlation between antibody and infection risk: The left vertical axis represents the relative infection risk compared to the placebo group, the horizontal axis represents the antibody level, the green line represents the correlation between antibody and infection risk, and the light green shade represents the 95% confidence interval. The pink histogram shows the distribution of antibody levels in the vaccinated group. The figure above shows the correlation of anti-Spike antibodies, and the figure below shows the correlation of antibody titers in live virus (Source: Reference 1)
The results of the study showed that after vaccination, if the anti-Spike antibody is higher than 40923AU/ml, or the live virus neutralization test titer is higher than 247, the risk of infection of the vaccinated person will be reduced to 20% of that of the placebo group, that is, the vaccine The effective rate reaches 80%; after vaccination, if the anti-Spike antibody is higher than 4446AU/ml, or the live virus neutralizing antibody titer can exceed 68, the vaccine effective rate will reach 50%.
Immunological protection related indicators VS immunological surrogate endpoint
This is the world’s first study on the immunological protection related indicators of the COVID-19 vaccine. Although it has not been officially published after peer review, Nature magazine has reported and discussed it.

Screenshot of Nature’s official website
To understand the significance of this research, we first need to understand two concepts: “Immunological Protection Related Indexes” and “Immunological Surrogate Endpoints.”
“Immunological protection-related indicators” refer to immune indicators that are related to the protective efficacy of vaccines and are relatively easy to measure, such as the level of neutralizing antibodies and T cell response in peripheral blood. The higher the level of these indicators, the lower the risk of infection, which means the stronger the protective efficacy of the vaccine.
Looking further, if it is possible to determine the level of these immune indicators that can effectively prevent infection, the protective efficacy of the vaccine can be predicted by testing these indicators, without the need to monitor the infection rate. Such immunological protection-related indicators are called “immunological surrogate endpoints” indicators for vaccine clinical trials.
At present, most vaccines use peripheral blood antibody levels as “immunological protection-related indicators.” The most common is to detect anti-HBs antibodies after inoculation with hepatitis B vaccine, which is considered effective when reaching 10mIU/ml; while the influenza vaccine uses a hemagglutination inhibitory antibody titer of 40 as the threshold.
According to the regulations of the European Medicines Agency, the influenza vaccine is replaced every year. As long as 70% of the 18-60 year-old vaccinators have a 40-fold dilution of the hemagglutination inhibitor antibody, this vaccine can be used; at the same time, the new Vaccine manufacturers use standard technology to produce inactivated influenza vaccines, and only need to assess the subject’s immune response, and there is no need to organize large-scale protective trials.
So, can the COVID-19 vaccine also choose the peripheral blood antibody level as the “immunological surrogate endpoint” like other vaccines?
This study, launched in June, gave a preliminary and definite answer: in the Oxford/AstraZeneca COVID-19vaccine, the level of neutralizing antibodies in the peripheral blood of the vaccinated person can be used as an “immunological protection-related indicator.”
However, there is still a long way to go from “immunological protection-related indicators” to “immunological surrogate endpoints.”
First of all, the confidence interval of the level of antibody protection obtained in this research paper is very wide, the accuracy is insufficient, and it is difficult to be directly used for clinical indicators. It is still necessary to further increase the sample size of subjects.
Secondly, it is best to use a globally uniform experimental method for the “surrogate endpoint of immunology”. In this study, the research team used the serum pool standard of recovered patients provided by WHO as a reference, and the experimental methods used were chemiluminescence and virus neutralization experiments. However, virus neutralization experiments are cumbersome and difficult to carry out on a large scale, and the antibody detection methods approved for use around the world vary greatly, and it still takes a lot of time to distribute standard products and establish uniform detection standards.
What does research mean?
After talking about this research, let’s go back to the two questions mentioned at the beginning of the article:
First, after receiving the COVID-19 vaccine, how can I confirm whether I am effectively protected?
An important significance of this latest study is that it confirms that the level of neutralizing antibodies in peripheral blood can be used as an “immunological protection-related indicator.”
However, another important point that must be drawn is that the results of this study do not mean that the current hospital’s COVID-19 antibody test can be used as a reference for the effectiveness of COVID-19 protection.
To put it more succinctly: After being vaccinated with the COVID-19 vaccine, it is not yet possible to judge whether one has been successfully immunized based on antibody testing.
We have just mentioned that the antibody detection methods used in different regions of the world vary greatly. Take some countries’s existing chemiluminescent antibody detection as an example, which detects the levels of anti-RBD and anti-N IgM antibodies and IgG antibodies.
The neutralizing ability of IgM antibodies is very weak. Therefore, a positive IgM antibody does not mean that neutralizing antibodies have been produced. The measured value of IgG antibody is the sum of anti-RBD and anti-N antibodies, and anti-N antibodies are not neutralizing antibodies. Therefore, positive IgG antibodies cannot accurately reflect the level of anti-RBD antibodies, let alone the level of neutralizing antibodies. .
On the other hand, a positive neutralizing antibody does not necessarily mean that you will be free from infection. This is because the standard for antibody positive is generally lower than the “immunological surrogate endpoint”. For example, the neutralizing antibody positive standard for inactivated vaccines is that the titer of the live virus neutralization test is greater than 8, which also makes the antibody conversion rate of most vaccines possible It reaches more than 95%, but the effective rate of preventing symptomatic infection is lower than the conversion rate.
At present, the new coronavirus antibody tests we can carry out are all for the purpose of diagnosing infection, but cannot verify the effect of the vaccine.

Screenshot of FDA official website
Let’s turn to the second question: Can the new coronavirus vaccine be clearly effective without a phase III trial?
Let me start with the conclusion: Generally speaking, when this kind of research becomes more and more perfect, we may be able to shorten the vaccine trial time and respond more quickly to the challenge of mutant strains.
To discuss this issue, another limitation of this study needs to be mentioned: the subjects were all vaccinated with Oxford/AstraZeneca vaccines, but different types of COVID-19 vaccines are different, whether the level of peripheral blood antibodies can be used as other COVID-19 vaccines The “Immunological Protection Relevance Index” of China needs more research.
For example, in May of this year, researchers from the University of New South Wales in Australia published a study in Nature Medicine. By analyzing the clinical trial data of seven COVID-19 vaccines, including Coxing, they initially discussed the level of neutralizing antibodies in peripheral blood. The immunological protection related indicators of the COVID-19 vaccine.
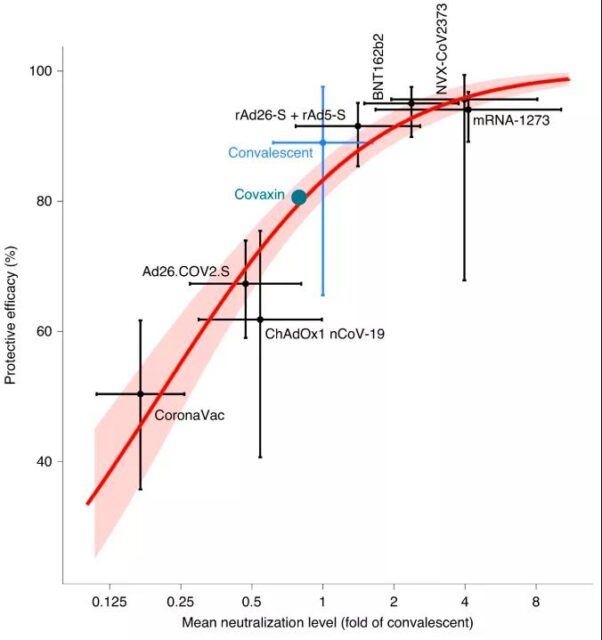
There is a correlation between the level of neutralizing antibody and the protective efficacy of the vaccine: the horizontal axis is the average neutralizing titre of the subjects in the phase I and II trials divided by the average titre of the recovered patients, and the vertical axis is the vaccine preventive effect in the phase III trial. For the protective efficacy of symptomatic infection, the vaccine brands from bottom left to top right are Coxing, AstraZeneca, Johnson & Johnson, India Covaxin, Russian Satellite V, Pfizer, Novavax, Moderna. The blue cross represents the effectiveness of neutralizing antibodies in the survivors to prevent re-infection. Cross-shaped error bars indicate the 95% confidence interval (Source: Reference 3)
However, this study only used the neutralizing antibody levels of subjects in phase I and II clinical trials to analyze the correlation with the protective efficacy of phase III, and did not monitor in the same subject population; in addition, this study The seven clinical trials of the meta-analysis come from different countries, and there are slight differences in the diagnostic criteria for symptomatic infections, and the experimental methods for the determination of neutralizing antibodies are also different. Therefore, it cannot be regarded as a strict immunological protection relevance certificate.
In general, this research is still at a very preliminary stage and is less important than the latest Oxford/AstraZeneca vaccine research. Different types of COVID-19 vaccines are likely to explore completely different immunological alternative endpoints. For this proposition, we need more research to discuss in the future.
In the future of the COVID-19 epidemic
When talking about this research, we mentioned the “future” many times. Where is the future of the COVID-19 epidemic? In the past year and a half, humans have tried to answer this question countless times.
On July 8, Nature’s opinion article once again envisaged three different future scenarios for the COVID-19 epidemic:

Screenshot of Nature’s official website
The first scenario is the unlikely future: the new coronavirus will eventually become similar to other human coronaviruses, and the impact of the disease is far less than that of influenza or SARS. But at present, it is difficult for us to predict whether the new coronavirus will reduce its toxicity in the future evolution process.
The second scenario is the most worrying future: we have not been able to quickly control the epidemic, and we need to continue to face the emergence of a large number of infections and the further evolution of the new coronavirus. Vaccines and past infections may provide long-term herd immunity, but this requires us to vaccinate widely across the world, and we also need to accurately, timely, and comprehensively monitor the epidemic for a long time.
The third scenario is the most likely future: the COVID-19 epidemic is gradually becoming an epidemic and becoming a seasonal disease similar to influenza. Therapies such as monoclonal antibodies can effectively control the progress of the COVID-19 pneumonia disease, and the infection level is equivalent to influenza or even more than influenza. low. However, this does not mean that we can take it lightly-influenza, in non-pandemic years, will also cause 250,000 to 500,000 deaths each year.
On July 12, the United Kingdom officially confirmed that it will officially lift the restrictions on epidemic prevention and isolation on the 19th of this month. “The COVID-19 epidemic is far from over, and it will certainly not end before July 19,” British Prime Minister Boris Johnson said. “Although we are sad, we must allow ourselves to accept more deaths caused by the COVID-19 epidemic.”
News screenshot
In China today, the vast majority of people have rarely felt the negative impact of the COVID-19 epidemic on their daily lives, but when we look at the world, the concept of “the COVID-19 virus will coexist with mankind for a long time” has gradually been accepted by more people.
With this concept in mind, we once again return to the study of “Immunological Protection Related Indexes”. What is the significance of it?
It must be admitted that we still have a long way to go from “immunological protection-related indicators” to “immunological surrogate endpoints.” However, in the near future, if we can determine the immunological surrogate endpoint of the COVID-19 vaccine and design corresponding testing methods, medical institutions can more accurately and conveniently determine the immunization effect of vaccinators, and timely supplement the booster shots for people with low immunity. ; Government departments can also learn more accurately the immunization level of local residents and use this as a basis to formulate public health policies.
On the other hand, “immunological surrogate endpoints” can also help us deal with mutant strains more quickly.
Taking the Oxford/AstraZeneca vaccine as an example, the vaccinated sera had an average neutralization titer of 306 against the live virus of the initial strain of the COVID-19 epidemic, and an average neutralization titer of 131 against the Alpha strain (first discovered in the UK). Only 71 for the Delta strain (first discovered in India). The decrease in the neutralization titer of the vaccinated against the mutant strain means that the protective efficacy of the vaccine is reduced.
In the near future, if we can determine the immunological surrogate endpoint of the COVID-19 vaccine, vaccine developers will be able to quickly assess the severity of the mutant strain and the extent of the mutant strain’s impact on the vaccine. A new vaccine with a replacement strain sequence can be approved only by evaluating the immunological indicators, eliminating the long wait for phase III trials and putting it into production and application as soon as possible.
So, how long do we have to go from a preliminary study to clinical application? Like “the future of the COVID-19 epidemic,” it is difficult to get a definitive answer to this question now. But what is certain is that human beings have never stopped exploring, which will help us to be more comfortable in all possible futures. (Planning: z_popeye, gyouza)
Expert Comments:
More and more evidence shows that the level of neutralizing antibody is still the most reliable immunological indicator in predicting the protection rate of the COVID-19 vaccine.
In fact, neutralizing antibodies, as a relevant indicator of immune protection, have been widely used in influenza vaccines, and they are also mentioned in the article. Influenza vaccines have immune escape due to antigenic drift, and the vaccines need to be updated based on the seasonal epidemic strains predicted every year. In the absence of conditions for re-launching clinical Phase III trials, neutralizing antibody levels are used as the surrogate endpoint.
At present, mutant strains of the new coronavirus have also appeared. Therefore, whether the protection rate of the vaccine against the new strain still needs to be tested in phase III, which has become a problem that has to be faced. If it can pass the relevant indicators of immune protection, that is, neutralizing antibodies To establish an immunological surrogate endpoint, it can greatly improve the efficiency of vaccine development.
In fact, this step is much more complicated, including complex research design (such as human challenge trials, randomized controlled trials with research indicators as clinical endpoints, observational studies, and extrapolation of animal experiments, etc.) and experimental methods (ROC curve method, Continuous method and threshold method, etc.), the results obtained by different research strategies may be biased, and more importantly, a large number of samples are required. Therefore, for the COVID-19 vaccine, the necessity and difficulty of setting immunological alternative endpoints are currently in progress. face the challenge.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



