First non-vaccine drug was authorized for emergency use by FDA
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
First non-vaccine drug was authorized for emergency use by FDA
First non-vaccine drug was authorized for emergency use by FDA. To prevent COVID-19, the first non-vaccine drug was authorized for emergency use by FDA. It is a drug used by former U.S. President Trump.
In the strategy of preventing new coronavirus infection, vaccines are the first choice. However, full vaccination takes time, at least 5 weeks to complete.
If you have not been vaccinated, or people with poor immunity,
But in close contact with the infected person,
Is there any way to prevent COVID-19?

On July 30, 2021, the US Food and Drug Administration (FDA) urgently authorized Regeneron‘s SARS-CoV-2 antibody REGEN-COV to prevent COVID-19 infection (PEP) after exposure.
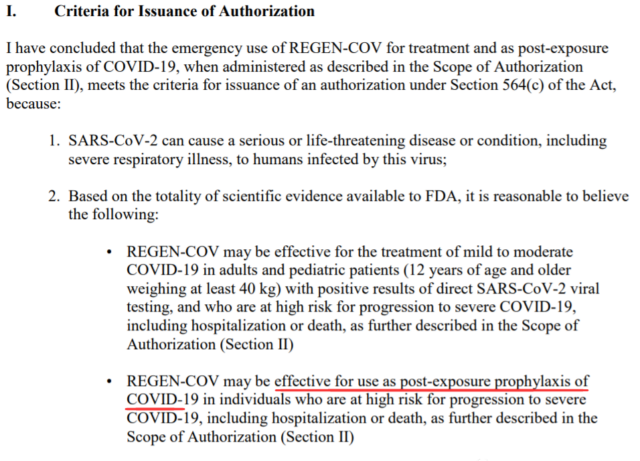
This EUA is limited to post-exposure prevention for people at high risk of progression, and cannot be used for non-exposure prevention; non-exposure prevention mainly relies on vaccines.
In a further explanation, the FDA defines people at high risk of progression as “people who have not been fully vaccinated or have a poor immune response.”

This EUA is of great significance for protecting high-risk groups, especially medical staff.
And REGEN-COV is still used to treat mild and moderate COVID-19 at the same time, and it is also the drug that former US President Trump used immediately after he was diagnosed with COVID-19.
The authorization now includes: post-exposure prophylactic treatment for people at high risk of developing severe COVID-19, these people are not fully vaccinated or are not expected to have an adequate immune response to vaccination, and have been exposed to SARS-CoV-2 infection Or because the infection occurs in the same institutional environment (such as a nursing home or prison), the risk of contact with an infected person is high.
For those who need repeated administration due to continuous exposure, REGEN-COV can now also be administered once a month. This new indication for people 12 years and older is in addition to the previously authorized treatment of ambulatory COVID-19 patients. REGEN-COV is not a substitute for vaccination to fight COVID-19, nor does it authorize pre-contact preventive treatment to prevent COVID-19.
It is worth mentioning that REGEN-COV is the only COVID-19 antibody therapy in the United States that can be used for both treatment and post-exposure preventive treatment. REGEN-COV retains its effectiveness against multiple variants of interest. Currently, the use of REGEN-COV in the United States is rapidly increasing in response to the ongoing COVID-19 epidemic.
This authorization update is supported by data from a key phase 3 clinical trial (REGN-COV 2069). The trial showed that among close contacts (family members) of SARS-CoV-2 infected persons, a single subcutaneous injection of REGEN-COV reduced the risk of symptomatic COVID-19 infection by 81%.
Dr. George D. Yancopoulos, President and Chief Scientific Officer of Regeneron, said: “Today’s FDA authorization allows certain people at high risk of severe COVID-19 infection to receive REGEN-COV treatment after being exposed to the virus. This is the first authorization. This type of antibody therapy is used for this purpose. According to this authorization, the FDA has particularly emphasized the needs of people with weakened immune functions, including those taking immunosuppressive drugs, who may have insufficient immune response to vaccination and have been exposed to COVID-19 infection. People who are at high risk of exposure due to infection in the same environment in the institutional environment. Today, the FDA has decided to expand the use of REGEN-COV in the post-exposure environment. This is a very beneficial step and we will continue Cooperate with the FDA because the agency is reviewing REGEN-COV in a wider population, including in pre-exposure prevention settings for people with immunocompromised functions, and in patients hospitalized with COVID-19.”
According to expert estimates, approximately 3% of the American population may have an incomplete immune response to COVID-19 vaccination due to immune damage or immunosuppressive drugs. This includes patients receiving chemotherapy, patients with hematological tumors such as chronic lymphocytic leukemia, patients receiving stem cells or hemodialysis, patients receiving organ transplants, and/or patients taking certain drugs that may weaken the immune response (e.g., mildew Phenolic acid esters, rituximab, azathioprine, anti-CD20 monoclonal antibody, Bruton’s tyrosine kinase [BTK] inhibitor). This authorization enables these people to use REGEN-COV to prevent infection after exposure and in certain institutional settings.
According to EUA’s post-exposure precautions, REGEN-COV can be administered by subcutaneous injection or intravenous infusion. For people who are not expected to have an adequate immune response after vaccination and are continuously exposed to SARS-CoV-2 for more than 4 weeks, after the initial dose is 1200 mg, REGEN-CoV 600 mg can be administered repeatedly every 4 weeks during the continuous exposure period.
REGEN-COV is an antibody cocktail therapy developed by Roche and Regeneron. Roche is mainly responsible for the development and supply outside the United States. REGEN-COV consists of two monoclonal antibodies (casirivimab and imdevimab), which target two independent, non-identical antibodies in the receptor binding region of the new coronavirus (SARS-CoV-2) spike protein (S protein). Overlapping sites have a synergistic effect, which can reduce the risk of virus mutation and escape, and protect the population from virus variants with mutations in the S protein. In addition, data from preclinical studies indicate that casirivimab and imdevimab retain neutralizing activity against key emerging variants.
In July of this year, REGEN-COV received the world’s first approval (trade name: Ronapreve) in Japan for the treatment of mild to moderate COVID-19 patients through intravenous infusion. In addition to Japan, REGEN-COV is currently authorized for emergency use or temporary pandemic use in more than 20 countries around the world (including the United States, the European Union, India, Switzerland and Canada. In the European Union, the European Medicines Agency (EMA) REGEN-COV Cocktail Therapy is undergoing a rolling review. Its subordinate organization Committee on Pharmaceuticals for Human Use (CHMP) has issued positive scientific opinions to support the use of REGEN-COV as a treatment option for the treatment of confirmed COVID-19 without supplementation. Patients with oxygen and high risk of developing severe COVID-19.
In the United States, REGEN-COV was granted an emergency use authorization (EUA) by the FDA in November 2020 for the treatment of direct SARS-CoV-2 virus test results positive, high risk of developing severe COVID-19 and/or hospitalization Treat children and adults with mild to moderate COVID-19 who are ≥12 years old and weigh ≥40 kg. REGEN-COV should be administered by intravenous (IV) infusion; when intravenous infusion is not feasible and will cause treatment delay, subcutaneous injection (SC) is an alternative method.
With the renewal of the US FDA authorization, when used for post-exposure prophylaxis, REGEN-COV 12OO mg (600mg casirivimab + 600mg imdevimab) can be administered by subcutaneous injection (4 injections) or intravenous infusion (in just 20 minutes). For people who are not expected to have an adequate immune response after vaccination and are continuously exposed to SARS-CoV-2 for more than 4 weeks, after the initial dose is 1200 mg, REGEN-COV 600 mg can be administered repeatedly every 4 weeks during the continuous exposure period.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



