FDA urgently authorized the therapy treating and preventing COVID-19
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA urgently authorized the therapy treating and preventing COVID-19
FDA urgently authorized the therapy treating and preventing COVID-19. The only therapy that can treat and prevent COVID-19 at the same time is here, and the FDA has urgently authorized it
It can not only treat COVID-19, but also prevent infection. How does this neutralizing antibody therapy perform?
Recently, the FDA expanded the scope of emergency authorization for REGEN-COV, allowing REGEN-COV to be used for post-exposure prophylactic treatment for people at high risk of developing severe COVID-19 [1]. Related research has also been published in NEJM for reporting.
In other words, REGEN-COV is currently the only antibody therapy in the United States that can be used for both the treatment and prevention of COVID-19.
REGEN-COV (previously known as REGN-COV2) is a double antibody therapy consisting of two neutralizing antibodies, casirivimab and imdevimab. It is worth mentioning that REGEN-COV is also the first combination therapy to be authorized by the FDA for emergency use. Another neutralizing antibody combination therapy with emergency authorization is the combination of etesevimab and bamlanivimab jointly developed by Junshi Biotechnology and Eli Lilly.
Reduce mortality and relieve symptoms of infection, The efficacy of REGEN-COV is excellent
Regeneron Pharmaceuticals stated in the 2021 American Thoracic Society International Conference (ATS 2021) that REGEN-COV can significantly reduce the risk of hospitalization or all-cause death of COVID-19 outpatients, quickly relieve symptoms of infection, and reduce viral load .
The study [2] was published in the journal medRxiv: The researchers divided 4057 subjects into three groups and randomly assigned them to receive placebo, REGEN-COV 1200mg or REGEN-COV 2400mg, and the test patients all had ≥1 severe COVID- 19 risk factors, the nucleic acid test was positive.
The results showed that REGEN-COV significantly reduced COVID-19-related hospitalization or all-cause mortality. The 2400mg dose group reduced by 71.3%, p<0.0001; the 1200mg dose group reduced by 70.4%, p=0.0024.
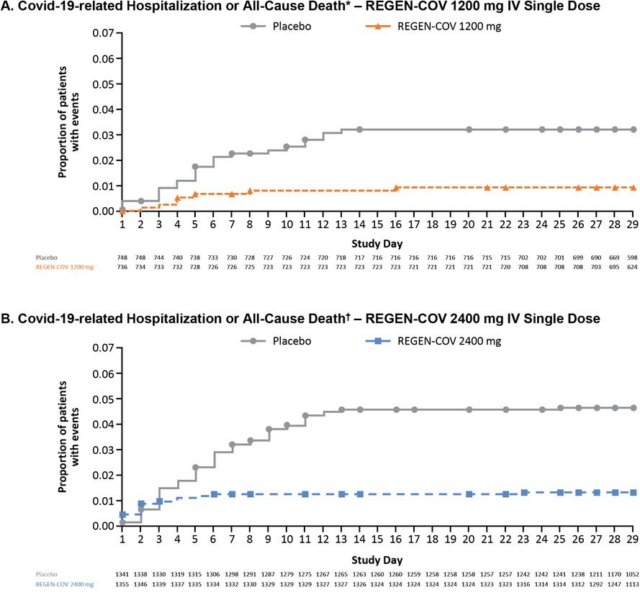
As shown in the picture of COVID-19-related hospitalizations or all-cause deaths, you can see that the REGEN-COV group curve (A yellow, B blue) is significantly lower than the placebo group.
The median time for symptom resolution in the REGEN-COV group was 4 days shorter than that in the placebo group. The median time for symptom resolution was 10 days for the 2400 mg and 1200 mg doses, and 14 days for the placebo group, p<0.0001.
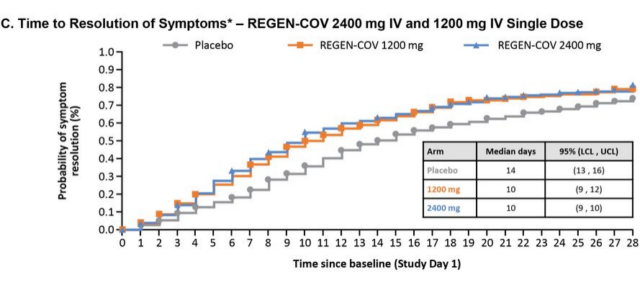
The graph shows the time curve of the number of people who have resolved. It can be seen that the proportion of the number of people who have resolved in the two doses of REGEN-COV (1200mg yellow, 2400mg blue) is higher than that of the placebo group (grey).
At the same time, compared with the placebo group, the REGEN-COV two-dose group can reduce the viral load faster; the incidence of adverse events caused by 1200mg is 1.1%, 2400mg is 1.3%, and the placebo group is 4.0 %.
In general, this study proved that intravenous REGEN-COV has a certain therapeutic effect on outpatients with COVID-19, and its safety is also guaranteed.
Poor vaccine protection, REGEN-COV can be used as a new preventive medicine
After determining the therapeutic effect of REGEN-COV, Regeneron conducted further research on the preventive and protective effects of REGEN-COV and other modes of administration. The test results of subcutaneous injection of REGEN-COV to prevent new coronavirus infection were published on August 4 In NEJM[3]:

Studies have shown that REGEN-COV can reduce the risk of infection in people who are not infected after exposure to the virus, and it has once again proven that REGEN-COV can reduce the duration of symptoms and the duration of high viral loads.
The study divided 1505 subjects into two groups randomly and injected REGEN-COV 1200 mg or placebo respectively. The subjects’ RT-qPCR test and serological test were negative, and they are expected to live with people with COVID-19 infection At least 28 days.
The results of the study showed that for symptomatic infections, the infection rate in the REGEN-COV group was 1.5% (11/753) and the placebo group was 7.8% (59/752), the relative risk was reduced by 81.4%, and the odds ratio was 0.17, P< 0.001.
For all symptomatic and asymptomatic infections, the infection rate in the REGEN-COV group was 4.8% (36/753) and the placebo group was 14.2% (107/752), the relative risk was reduced by 66.4%, and the odds ratio was 0.31, P< 0.001.
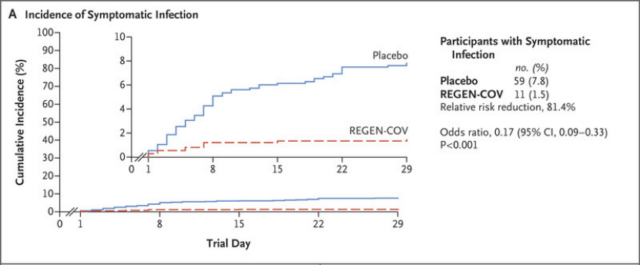
The graph shows the incidence of symptomatic infections. It can be seen that the REGEN-COV group (red) is significantly lower than the placebo group (blue)
Among patients diagnosed with symptomatic infection, the average time to symptom relief was 1.2 weeks in the REGEN-COV group and 3.2 weeks in the placebo group.
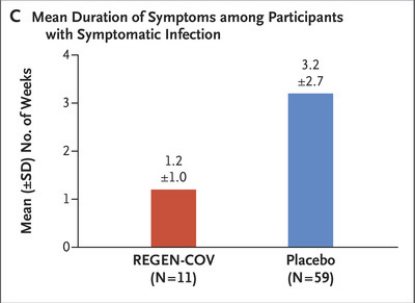
The graph shows the average duration of symptoms. It can be seen that the REGEN-COV group (red) is significantly lower than the placebo group (blue)
Among patients diagnosed with symptomatic infection, 1.6% (12/745) of patients with high viral load in the REGEN-COV group, 11.3% (85/749) in the placebo group, a relative risk reduction of 85.8%, and an odds ratio of 0.13 ; P<0.001.
The average duration of high viral load was 0.4 weeks in the REGEN-COV group and 1.3 weeks in the placebo group.
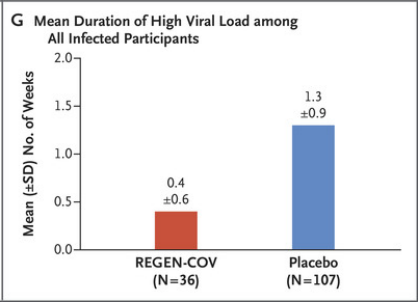
The graph shows the average duration of high viral load. It can be seen that the REGEN-COV group (red) is significantly lower than the placebo group (blue)
In general, subcutaneous injection of REGEN-COV can protect close contacts from being infected to a certain extent, while reducing the duration of symptoms and the duration of high viral loads in infected persons. At the same time, the study proved that subcutaneous injection of REGEN-COV is effective, has acceptable safety, and can reduce the consumption of medical resources required for intravenous infusion.
It should be emphasized that this emergency authorization of REGEN-COV is limited to prevention after exposure to the new coronavirus, and cannot be used for non-exposure prevention. In other words, when there are no new coronavirus infections around, you still have to get vaccinated.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



