Taiwan approved its own COVID-19 vaccine for emergency use
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
Taiwan approved its own COVID-19 vaccine for emergency use
- Israel new drug for COVID-19: EXO-CD24 can reduce deaths by 50%
- COVID-19 vaccines for children under 12 will be available soon
- Breakthrough infection of Delta: No difference from regular COVID-19 cases
- French research: ADE occurred in Delta variant and many doubts on it
- Deer herds in U.S. are heavily infected with the new coronavirus (COVID-19)
Taiwan approved its own COVID-19 vaccine for emergency use. Does the Medigen vaccine, which is urgently approved by “Immune Bridging” in Taiwan, really work?
Taiwan has accumulated 15,926 COVID-19 cases, of which 828 have died.
On August 23, 2021 (Monday), the COVID-19 vaccine named Medigen MVC-COV1901 was officially launched in Taiwan. It is expected that 60,000 people will be vaccinated this week.
On July 17, 2021, Medigen MVC-COV1901 vaccine was reviewed by the drug regulatory authority in Taiwan, China, and obtained an emergency use authorization (EUA) for use by adults over 20 years of age.
The review decision of Medigen MVC-COV1901 vaccine is shown in the figure below.
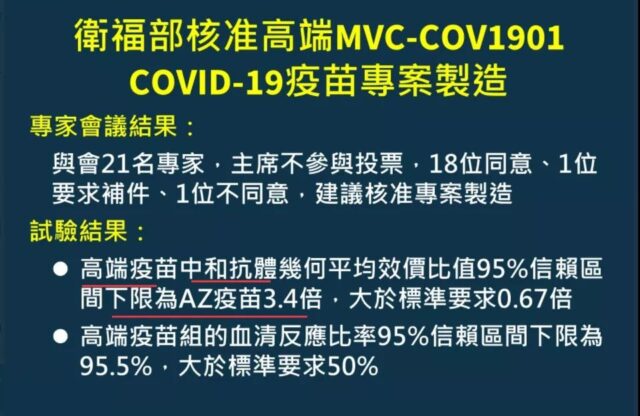
(Approval results announced in Taiwan. Source: Twitter)
Careful friends may find that the Medigen MVC-COV1901 vaccine does not mention the protection of the vaccine; instead, the neutralizing antibody GMT induced by the vaccine is 3.4 times that of the AstraZeneca (AZ) vaccine, and the seroconversion rate 95.5%; that is, “immuno-bridging” (immuno-bridging) will replace the results of phase 3 clinical trials on the market.
The “immune bridging” here refers to the prediction of vaccine protection in phase 3 clinical trials by comparing the level of neutralizing antibodies induced by the vaccine in early clinical trials.
To put it bluntly, this is a preterm vaccine that has not been validated by a complete clinical trial for effectiveness and safety.
Although we often say that antibody titers in early clinical trials can predict vaccine protection; this is limited to scientific research or investment considerations; and to apply it to COVID-19 vaccine approval, Medigen MVC-COV1901 vaccine is the first.
Of course, behind everything, there is a last resort to the truth.
The main reason why Taiwan region started to vaccinate this preterm vaccine was “there is no vaccine available.”
Because of the outbreak in India, the AstraZeneca vaccine originally ordered at the Serum Institute of India, the world’s largest vaccine center, has faced significant delays. In the face of highly infectious Delta map mutant strains, the current vaccination rate in Taiwan is less than 40%. Once COVID-19 spreads again, the consequences will be disastrous.
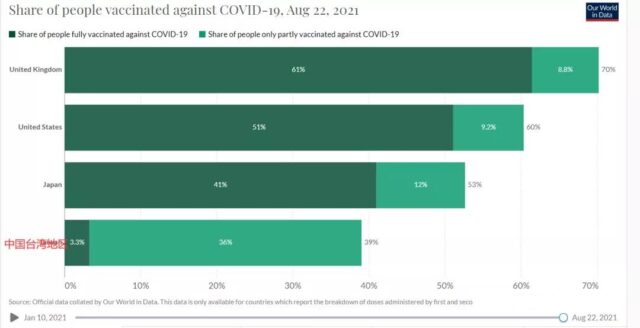
Does Medigen MVC-COV1901 vaccine work?
Because there is no phase 3 clinical trial results, it is impossible to give an accurate answer. However, judging from the results of early clinical trials of Medigen MVC-COV1901 vaccine, its approval also seems logical.
First, the Medigen MVC-COV1901 vaccine uses S-2P, a stable pre-fusion protein technology.
We have previously summarized the key to a successful vaccine: the rational design of the conformational epitope of the vaccine antigen + liposome nanoparticles to protect the stably expressed antigen is the core of the technology.
The antigen used in the Medigen MVC-COV1901 vaccine is exactly the S-2P stable pre-fusion protein technology of the Barney Graham team of the National Institutes of Health Vaccine Research Center (VRC), which is the two summarized by Dr. Fauci, Chief Scientist of the White House. One of the key technologies, and Graham is the official developer of vaccine antigen technology.
Dr. Fauci talked about the hero of the COVID-19 vaccine research and development. Why does she take credit for it?
Secondly, the adjuvant of Medigen MVC-COV1901 vaccine uses CyG 1018 adjuvant of American Dynavax company.
This adjuvant has excellent performance in enhancing immunogenicity, and it is also a product that defeated GlaxoSmithKline in the competition and became a clover COVID-19 protein vaccine adjuvant.
Third, high neutralizing antibodies were induced in early clinical trials.
In the phase 2 clinical trial, the neutralizing antibody induced by the 15ug dose of Medigen MVC-COV1901 vaccine was 1.51-1.94 times that of the recovered patient’s serum.
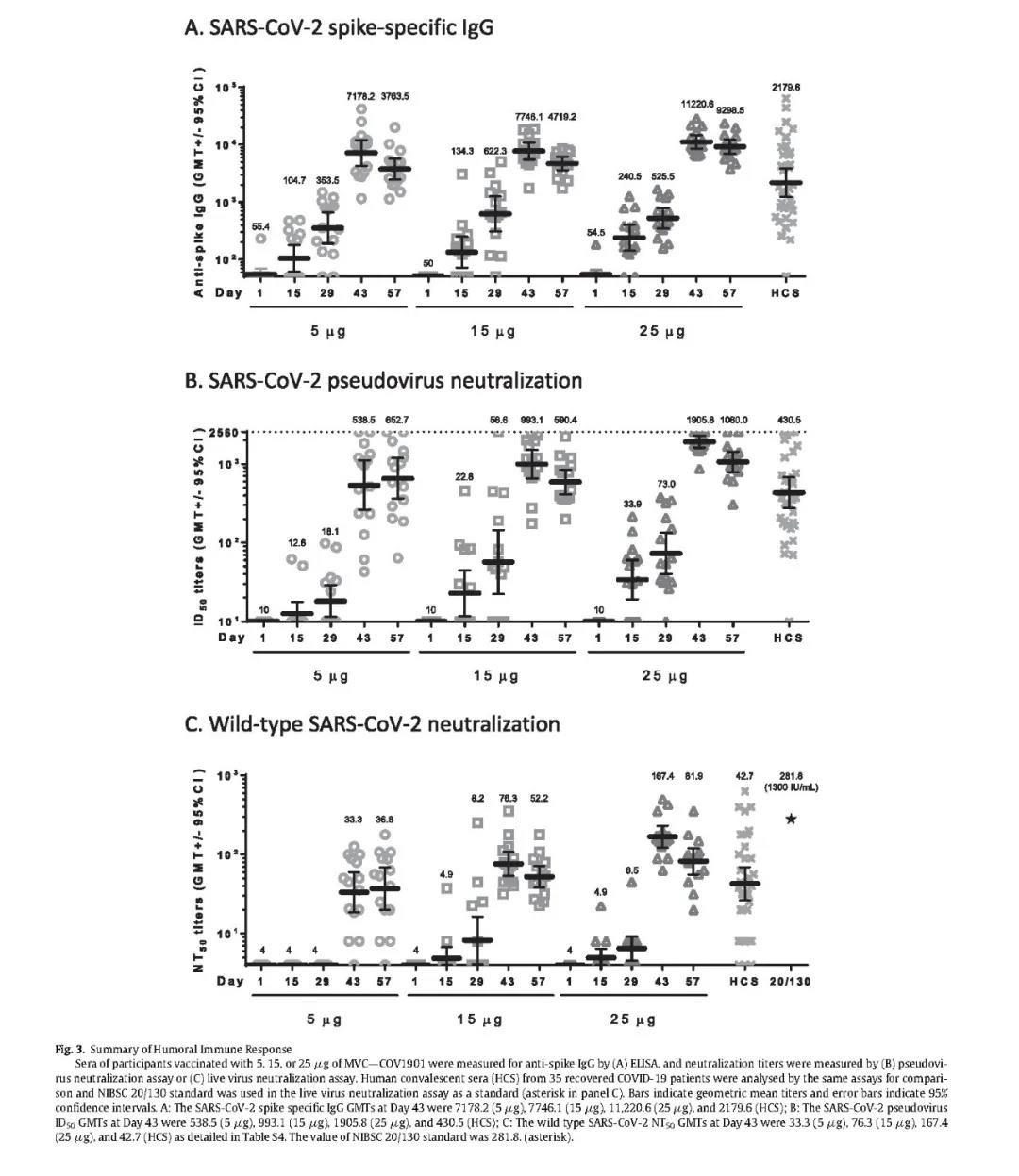
(Phase 1 clinical trial shows that Medigen MVC-COV1901 vaccine induces antibodies much higher than the serum levels of recovered patients)
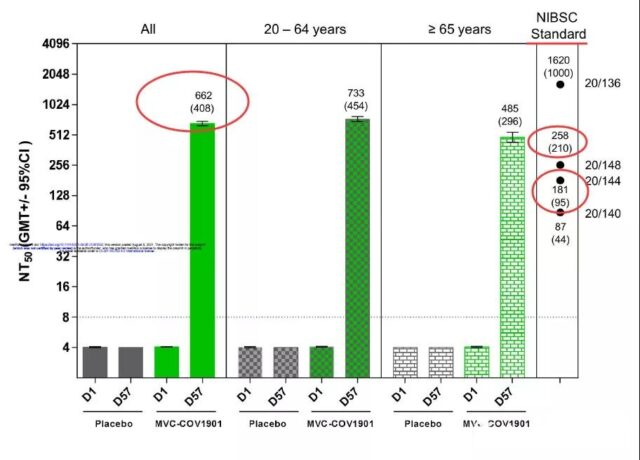
(In the phase 2 clinical trial, the neutralizing antibody induced by Medigen MVC-COV1901 vaccine was 1.51-1.94 times of the serum of recovered patients)
Fourth, in early clinical trials, Medigen MVC-COV1901 vaccine demonstrated good safety.
From predictive models to clinical practice; Medigen MVC-COV1901 vaccine, which has obtained emergency use authorization through “immune bridge”, is leading the way. Although full of helplessness, it also provides new explorations for emergency plans in the event of a sudden epidemic.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



