FDA approved the first surgical robot for cochlear implantation
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA approved the first surgical robot for cochlear implantation
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FDA approved the first surgical robot for cochlear implantation
On October 5, the official website of iotaMotion announced that the FDA had approved the DeNovo classification request for its iotaSOFT cochlear implant robot.
Although surgical robots are usually reminiscent of multiple autonomous arms arranged on an operating table, iotaSOFT’s minimalist system focuses on the single task of implanting electrode arrays in the inner ear, which is more precise than a human hand and does not require heavy hardware.
iotaSOFT is the world’s first and only robot-assisted implant technology that helps surgeons place cochlear implants by controlling the speed of the implant.
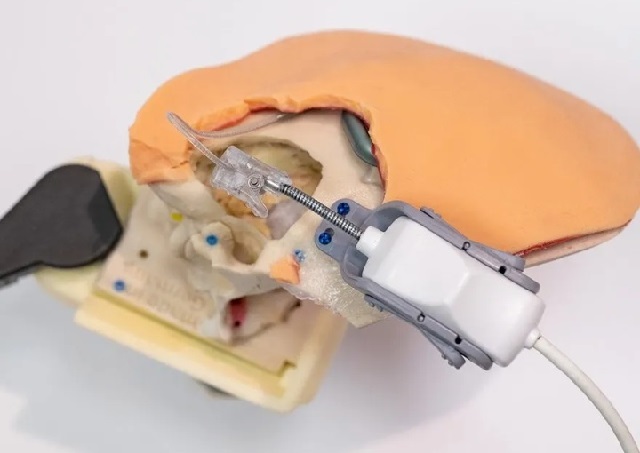
Image source: iotaMotion
“We are very pleased to launch the iotaSOFT implant system, which is the world’s first and only cochlear implant robot-assisted implant system that is commercially authorized by the FDA. It objectively provides precision and control that surpasses human capabilities.” said Christopher Kaufmann, MD, president and CEO of iotaMotion.
iotaSOFT is a small open platform robotic technology the size of a thumb. It is compatible with various implants and seamlessly integrates into existing surgical procedures. It is expected to become a fixed device in the field of cochlear implants. It is worth mentioning that surgeons control the precise movement capabilities of robot-assisted implants while still using their technical skills, training, and experience.

Image source: iotaMotion
By slowing down the operation speed and giving the surgeon more control, iotaSOFT helps the patient maintain the remaining hearing by limiting the damage to the fragile cochlea caused by the operation itself.
According to iotaMotion, research has shown that when the implant is inserted manually, the force used will surge when the electrode is pushed through the last few millimeters of the sensitive area of the ear. iotaMotion estimates that 15% to 50% of all patients receiving cochlear implants report additional loss of their natural hearing.
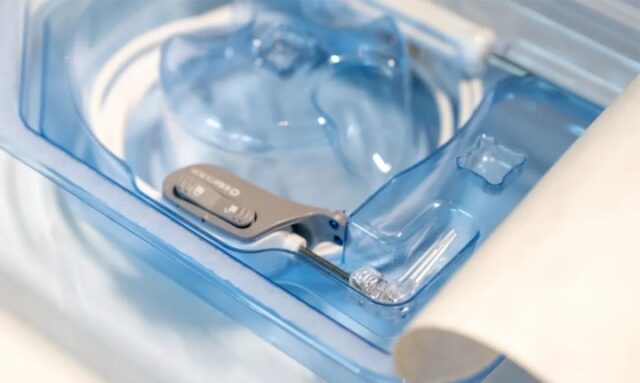
Image source: iotaMotion
“In the past, people could only place cochlear implant electrode arrays manually with tweezers and other manual instruments.
With the advent of robot-assisted systems such as iotaSOFT, we are able to place electrodes on the cochlea with the help of more precision and stability provided by robot assistance.
In the delicate and tiny structure.” said Marlan Hansen, MD, medical director of iotaMotion and head of the Department of Otorhinolaryngology at the University of Iowa. “Obviously there is an opportunity to improve efficacy, optimize the maintenance of existing functions, and provide consistent results for surgeons and patients in the United States and around the world.”

Image source: iotaMotion
“We look forward to working closely with surgeons, hospitals and implant partners to expand the wide application of the iotaSOFT system. The goal is to create a new standard of care for cochlear implants,” said Eric B. Timko, executive chairman of the board.
According to iotaMotion, it plans to introduce its device to healthcare professionals in the coming months through a controlled commercialization timetable under FDA de novo approval.
iotaMotion is a private company headquartered in Iowa, USA, which is developing robotics technology that focuses on personalized hearing loss treatment. The company’s solution aims to standardize cochlear implants, provide unprecedented control in surgical care scenarios, and expand surgeons and patients’ access to cochlear implants.
FDA approved the first surgical robot for cochlear implantation
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



