NEJM: Dexamethasone does not increase infection risks at surgical site
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
NEJM: Dexamethasone does not increase infection risks at surgical site
NEJM: Dexamethasone does not increase infection risks at surgical site. Dexamethasone is a cheap and effective long-acting glucocorticoid used to prevent and treat postoperative nausea and vomiting.
However, there are also concerns that dexamethasone will increase the risk of infection at the surgical site, especially in susceptible people such as diabetic patients. On May 6, 2021, the Tomás B. Corcoran team of the Royal Perth Hospital in Australia published the results of the perioperative dexamethasone and infection (PADDI) trial in the New England Journal of Medicine.
The study included 8880 non-emergency, Patients undergoing non-cardiac surgery to assess the impact of dexamethasone on the risk of infection at the surgical site.
Methods:
This is an international multicenter, randomized, placebo-controlled, triple-blind (patient, anesthesiologist, and evaluator) non-inferiority trial. 8880 patients were included in the trial. The final modified intention-to-treat population included a total of 8725 patients (4372 cases in the dexamethasone group and 4353 cases in the placebo group), of which 13.2% (576 cases in the dexamethasone group and 572 cases in the placebo group) suffered diabetes.
Inclusion conditions:
Adult patients, ASA classification I-IV, undergo elective or non-emergency, non-cardiac surgery, skin incision length is more than 5cm, receive general anesthesia, the expected operation time is at least 2h, and the postoperative hospitalization is expected to be at least 1 night.
Exclude conditions:
The patient intends to undergo a limited-time surgery; the expected total incision length is ≤5cm; the incision infection is related to the primary infection (for example, the infection related to the prosthesis); the intraoperative need to use dexamethasone; there is poorly controlled diabetes (defined as glycosylation) The hemoglobin level is >9.0%).
Program implementation:
Within 5 minutes after induction of anesthesia (before surgical skin incision), the attending physician of anesthesiology department received an intravenous injection of 8mg dexamethasone or placebo (2ml, random letters on the bottle body). This dose is very common in practice, and when used as an antiemetic, it has an additional analgesic benefit over the 4 mg dose. Other aspects (including preventive use of antibiotics, blood sugar level management, and medication treatment for diabetic patients) follow the local hospital’s routine and established guidelines. Non-experimental glucocorticoids are forbidden to be used within 30 days after surgery.
Further exclusion:
Patients who did not receive dexamethasone or placebo or received open-label dexamethasone (or other glucocorticoids) during or within 30 days after surgery.
Main results:
According to the definition of CDC in the United States, the infection of the surgical site within 30 days after the operation includes three types: shallow incision, deep incision and inter-organ infection.
Secondary results:
1) Including superficial, deep and inter-organ infections within 30 days after surgery, respectively, to be assessed;
2) Consider the deep and inter-organ infections within 90 days after the operation of patients implanted with prosthetic materials;
3) Other infections (including urinary tract infections, pneumonia, catheter-related infections and sepsis) occur within 30 days after surgery;
4) The quality of recovery on the 1st and 30th days (using the 15-item Quality of Recovery [QoR-15] scale for evaluation, the score ranges from 0 to 150, the higher the score, the better the quality of recovery);
5) Chronic postoperative pain at 6 months after operation;
6) Death or continuous new disability within 6 months after surgery.
Follow-up time:
Wake up room, 3 days after operation, at discharge, and 30 days and 6 months after operation. The scores of the QoR-15 scale (15-item recovery quality scale) were evaluated on the 1st and 30th day.
Results:
Main results:
In the modified intention-to-treat population, 354 (8.1%) of 4350 patients in the dexamethasone group had surgical site infections within 30 days after surgery, and 394 (9.1%) of 4328 patients in the placebo group Surgery site infections occurred within 30 days after surgery (adjusted for diabetes risk difference, -0.9%; 95.6%[CI], -2.1~0.3), and the results were consistent with non-inferiority (risk ratio 0.89; 95.6%[ CI], 0.77~-1.03; non-inferiority P<0.001).
The results of the main analysis are consistent with the non-inferiority of the population of each program (risk difference, -0.9%; 95.6% [CI], -2.1~0.3; non-inferiority P<0.001) and the population after treatment (risk difference, 0.04 percentage points; 95.6%[CI], -1.2~1.2; non-inferiority P=0.001). These results differed the least in the sensitivity analysis after estimating the missing data and adjusting the test sites (Table 1).
Table 1 Analysis of the results of the modified intention-to-treat population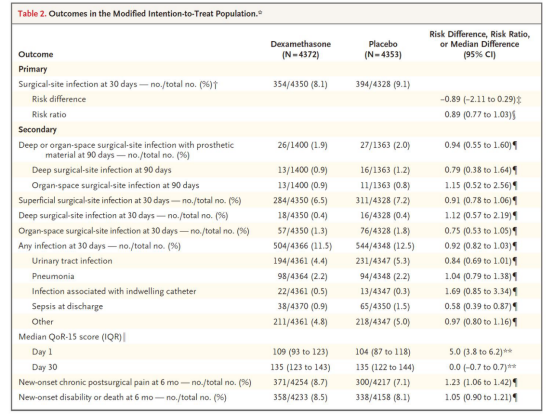

Secondary results:
The incidence of superficial, deep, or inter-organ infections assessed separately in the two groups was similar. In the two groups of patients, whether they were considered individually or in combination, the incidence of infection at the surgical site of the deep or inter-organ space within 90 days after the implantation of artificial materials was also similar.
0.9% of patients in the dexamethasone group and 1.5% of patients in the placebo group developed sepsis on the day of discharge (hazard ratio 0.58; 95% confidence interval, 0.39-0.87).
The median QoR-15 scores on day 1 were 109 and 104, respectively (median difference 5.0 points; 95% confidence interval, 3.8 to 6.2). The incidence of chronic pain at 6 months postoperatively was 8.7% in the dexamethasone group and 7.1% in the placebo group (hazard ratio 1.23; 95% confidence interval, 1.06 to 1.42).
The third level and safety results:
The incidence of nausea and vomiting within 24 hours after surgery was 42.2% in the dexamethasone group and 53.9% in the placebo group (hazard ratio 0.78; 95% confidence interval, 0.75 to 0.82).
Hyperglycemia occurred in 22 (0.6%) of 3787 patients in the dexamethasone group and 6 (0.2%) of 3776 patients in the placebo group.
The median difference in blood glucose level between the preoperative blood glucose level and the maximum blood glucose level recorded on the 2nd day after surgery was 3.6 mmol/l (interquartile range, 2.5 to 4.9 [65 mg/dl; interquartile range, 45- 88]) and 2.4 mmol/l (interquartile range, 1.4-3.6 [43 mg/dl; interquartile range (25-65]).
Nineteen patients (0.5%) in the dexamethasone group and 4 patients (0.1%) in the placebo group received insulin therapy for non-diabetic patients (Figure 1).
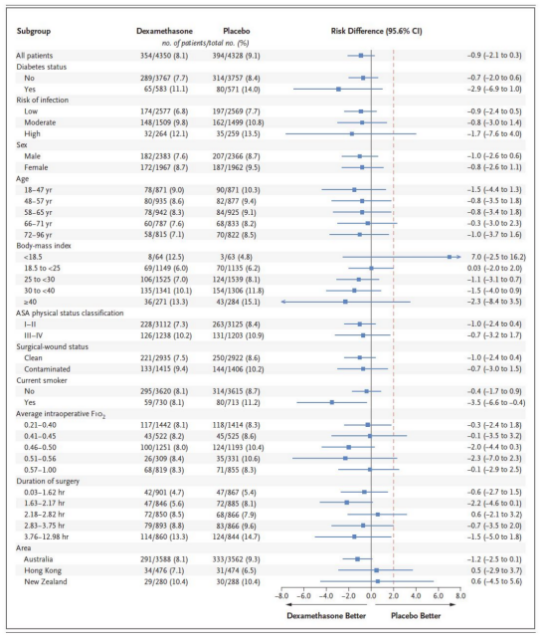
Figure 1 Subgroup analysis results of the two groups of patients
Conclusion:
Compared with placebo, patients with non-emergency, non-cardiac surgery were given 8 mg after induction of general anesthesia
Expert comments:
Dexamethasone has anti-inflammatory, anti-allergic and anti-shock effects. It is mainly used in the perioperative period to prevent nausea and vomiting and improve postoperative analgesia. Dexamethasone has a plasma half-life of 190 minutes and a tissue half-life of 72 hours. It is a long-acting glucocorticoid. Its plasma protein binding rate is lower than other glucocorticoid drugs.
The anti-inflammatory activity of 0.75 mg dexamethasone is equivalent to 5 mg prednisone. Previous studies have suggested that long-term use of glucocorticoids is associated with increased risk of surgical site infections and poor wound healing.
In recent years, many guidelines recommend the use of dexamethasone during the perioperative period to prevent postoperative nausea and vomiting and improve patient analgesia. In view of the 72h half-life of dexamethasone, which can cause changes in the expression of genes related to initial immune cells, the risk of perioperative single-dose administration of dexamethasone and surgical site infection has aroused academic research interest and has become a hot issue.
In response to this question, although there have been related studies in the past, there is no definitive answer due to reasons such as small sample size, insufficient dosage, and exclusion of diabetic patients. This study included 8880 non-emergency and non-cardiac surgery patients who were given 8 mg dexamethasone after induction of anesthesia, which fully overcomes the deficiencies of previous research designs. The results of the study suggest that the incidence of surgical site infections in the two groups was similar.
Based on the existing research evidence, a single dose of dexamethasone during the perioperative period does not increase the incidence of postoperative wound infection and prolonged wound healing time. Although the use of dexamethasone during the perioperative period can temporarily increase the blood glucose level within 24 hours after the operation, it has no adverse effect on the patient’s perioperative outcome.
The study has no shortage of design flaws. First, this study is based on modified intention-to-treat, and there are cases where the research plan is not followed. Second, this study failed to collect perioperative factors that may affect the risk of infection at the surgical site, such as the type of gastrointestinal surgery, preoperative skin and bowel preparation methods, and the type of perioperative antibiotics used. In addition, although this study claims that the dexamethasone group can reduce the incidence of postoperative nausea and vomiting, the incidence of nausea and vomiting within 24 hours after surgery is still as high as 42.2%, which is worthy of further analysis.
(source:internet, reference only)
Disclaimer of medicaltrend.org



