2022 ESMO: Treatment Guidelines for Advanced Thyroid Cancer Updates
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
2022 ESMO: Systematic Treatment Guidelines for Advanced Thyroid Cancer Updates
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
2022 ESMO: Systematic Treatment Guidelines for Advanced Thyroid Cancer Updates.
On April 28, 2022, the European Society for Medical Oncology (ESMO) updated the content of the clinical practice guidelines for the systemic treatment of advanced thyroid cancer.
In recent years, new targeted therapy drugs have been approved for the treatment of advanced/metastatic thyroid cancer.
Based on the opinions of a multidisciplinary expert panel on thyroid cancer, the guideline provides up-to-date treatment recommendations for thyroid cancer and summarizes the selection and order of use of these treatment options. The Rubik’s Cube has organized the key content.
Recommendations for systemic treatment of advanced differentiated and poorly differentiated thyroid cancer
- In the United States, cabozantinib is available for adults and adolescents ≥12 years of age with radioactive iodine (RAI)-refractory advanced/metastatic differentiated thyroid cancer (DTC) that has progressed after multikinase inhibitor (MKI) therapy [I,A] (FDA approved, EMA not approved).
- In Europe, Selpercatinib can be used in adult patients with advanced RET fusion-positive DTC who have already received MKI therapy (sorafenib, lenvatinib, or both) [V,B] (EMA approved, the FDA has not approved this indication).
- In the US, Serpa is an option for adults and adolescents ≥12 years of age with RAI-refractory advanced RET fusion-positive DTC, regardless of MKI prior to sorafenib, lenvatinib, or both Treatment with tinib [V,B] (FDA approved, EMA not approved for this indication).
- In the U.S., Pralsetinib is indicated for adults and adolescents ≥12 years of age with RAI-refractory advanced/metastatic RET fusion-positive DTC requiring systemic therapy [V,B] (FDA approved, EMA not approved) .
- Larotrectinib is indicated for adults and adolescents ≥12 years of age with advanced NTRK fusion-positive solid tumors that are not candidates for surgery and have progressed after treatment [V,B] (approved by both FDA and EMA).
- Entrectinib is indicated for adults and adolescents ≥12 years of age with metastatic or unresectable NTRK fusion-positive solid tumors that have progressed despite standard therapy [V, B] (approved by both FDA and EMA).
- Before systemic therapy for advanced DTC patients, genetic testing should be considered to assist in the decision-making of individualized treatment plans, and next-generation sequencing (NGS) testing is recommended as the first choice [III, C].
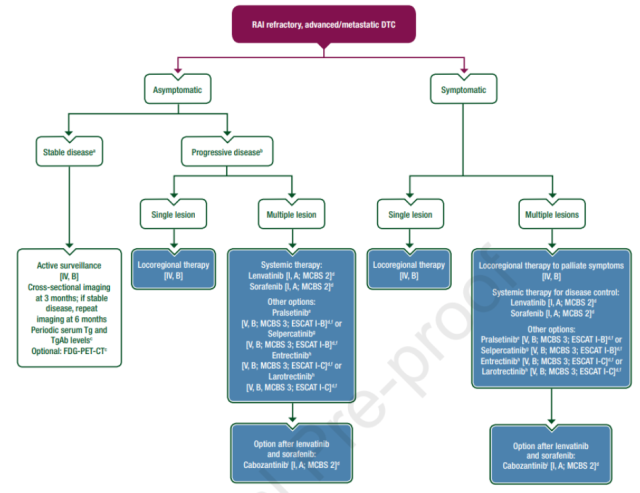
Figure: Treatment recommendations for patients with RAI-refractory advanced/metastatic DTC
Systemic treatment recommendations for advanced anaplastic thyroid cancer (ATC)
- • In patients with locally advanced or metastatic ATC with BRAF V600E mutation, dabrafenib combined with trametinib is recommended [IV, B].
- • In the presence of other druggable mutations (RET and NTRK rearrangements), appropriate targeted therapy is recommended (see Recommendations for the treatment of DTC and poorly differentiated TC) [V,B].
- • Immunotherapy is an alternative when genetic testing reveals a non-druggable mutation. In a phase II trial of 42 patients with locally advanced/metastatic ATC, the overall response rate with the PD-1 inhibitor spartalizumab was 19% and the response rate in patients with positive PD-L1 expression was 29% [V,B ].
- • Other approaches are in clinical trials [V,B].
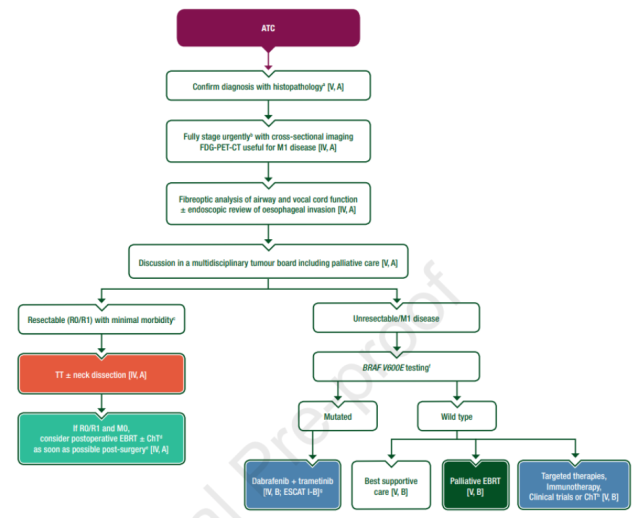
Figure: Treatment recommendations for patients with ATC
Systemic treatment recommendations for advanced medullary thyroid carcinoma (MTC)
- In Europe, serpatinib is available for adults and adolescents ≥12 years of age with advanced RET-mutant MTC requiring systemic therapy after cabozantinib and/or vandetanib [V,B] ( EMA approved, FDA not approved for this indication).
- In the United States, serpatinib is available for adults with advanced or metastatic RET-mutant MTC and pediatric patients ≥12 years of age who require systemic therapy [V,B] (FDA approved, EMA not approved for this indication).
- In the United States, pratinib is indicated for adults and children ≥12 years of age with advanced or metastatic RET-mutant MTC requiring systemic therapy [V,B] (FDA approved, EMA not approved for this indication).
- Prior to systemic therapy for patients with advanced/metastatic MTC, RET mutation gene testing is strongly recommended to assist in decision-making and individualized treatment. Allelic specific real-time PCR (allelic specific real-time PCR) or NGS detection is recommended as the first choice [III, A].
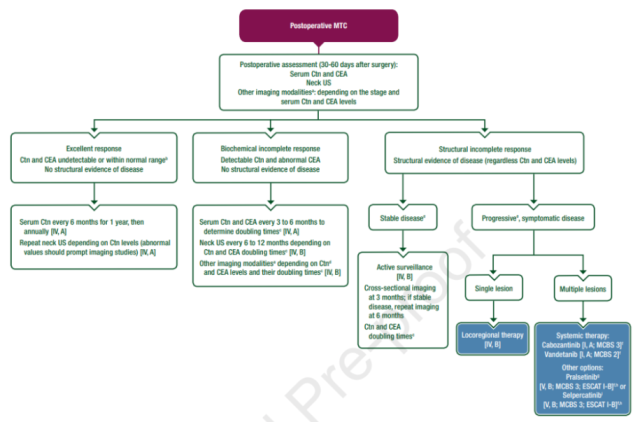
Figure: Recommendations for postoperative treatment of MTC patients
2022 ESMO: Systematic Treatment Guidelines for Advanced Thyroid Cancer Updates
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



