Omicron BA.4/5 escape vaccine has strong ability and the vaccine needs to be optimized
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Omicron BA.4/5 escape vaccine has strong ability and the vaccine needs to be optimized
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Omicron BA.4/5 escape vaccine has strong ability and the vaccine needs to be optimized.
The strong immune escape ability enables BA.4 and BA.5 to infect not only patients who recovered from delta and other mutants and those who received 3 doses of mRNA vaccine, but also other omicron mutants such as BA.1/2 who recovered. BA.4/5 has now become the dominant new coronavirus variant in the United States, Europe, South Africa and other places.
A paper published in the New England Journal of Medicine (NEJM) on June 23 showed that two weeks after inoculation with 3 doses of the mRNA vaccine BNT162b2, the neutralizing antibody titers against BA.4/5 decreased by 3.3 times compared with BA.1, and compared with BA.1. The original strain decreased by a factor of 21.
Early this morning, NEJM published online a communication article by the team of Academician Gao Fu from the Institute of Microbiology, Chinese Academy of Sciences.
The authors evaluated the sera of the inoculated SARS-CoV-2 original strain and omicron subunits after inoculation with 3 doses of inactivated vaccine, 3 doses of protein subunit vaccine ZF2001, or two doses of inactivated vaccine and 1 dose of ZF2001 booster by pseudovirus test. type of neutralizing antibody titers.
The results showed that the immune evasion ability of omicron BA.4/5 against the above two vaccines was similar to that of the mRNA vaccine BNT162b2, but the authors found that prolonging the interval between the second and third doses of ZF2001 increased neutralizing antibody titers against BA.4/5. Spend.
Neutralization of SARS-CoV-2 omicron variants induced by inactivated vaccine and ZF2001 vaccine
Omicron SARS-CoV-2 Neutralization from Inactivated and ZF2001 Vaccines
DOI: 10.1056/NEJMc2206900
In the third year of the Covid-19 epidemic, SARS-CoV-2 omicron variants swept the world and produced several subtype variants.
Currently, the infection rate of BA.2 is surpassing that of BA.1. In addition, BA.2.12.1 infections are rapidly increasing and already account for more than 50% of new infections in the United States. Therefore, the protective power of existing vaccines and the development of future vaccination strategies are of great concern.
In the present study, we evaluated the use of pseudovirus assays in vaccinated serum samples against the original strain (PT) of SARS-CoV-2 and against omicron subtype variants BA.1, BA.1.1, BA.2, Neutralizing antibody titers for BA.2.12.1, BA.3, BA.4 and BA.5.
Vaccines have received three doses of inactivated virus vaccine (one of two widely vaccinated in China: Clarifol and BBIBP-CorV), three doses of protein subunit vaccine ZF2001 (using the dimer receptor binding domain [RBD] ] as antigens) or two doses of Clarifol and ZF2001 boosters.
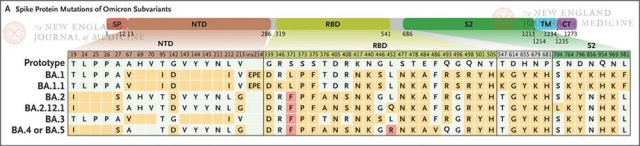
Figure 1. Spike protein mutations in variants of omicron subtypes.
The figure shows the spike protein mutations of omicron subtype variant strains BA.1, BA.1.1, BA.2, BA.2.12.1, BA.3, BA.4 and BA.5. (The last two subtype variants are grouped together because they share the same spike protein.) Amino acid differences (including substitutions, deletions, and insert) are highlighted in gold. The third amino acid at the same site is marked in red.
Deletion mutations are shown as blank squares with background color.
CT stands for C-terminal cytoplasmic domain, NTD stands for N-terminal domain, RBD stands for receptor binding domain, SP stands for signal peptide, S2 stands for S2 protein subunit, and TM stands for transmembrane domain.
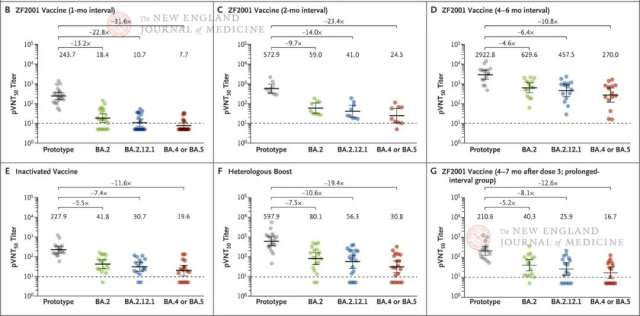
Figure 2. Neutralizing antibodies against omicron subtype variants induced by ZF2001 vaccine and inactivated virus vaccine.
Panels B to G show 50% pseudovirus neutralization titers (pVNT50) against the listed PT strains and omicron subtype variants BA.2, BA.2.12.1, BA.4 and BA.5.
Samples were grouped according to vaccine type and vaccination strategy. The numbers of these groups were as follows: 20 vaccinated in panel B, 9 vaccinated in panel C, 15 vaccinated in panel D, 16 vaccinated in panel E, and 19 vaccinated in panel F.
The 5 groups were sampled approximately 1 month after the last dose. Group 6 (Panel G) of 16 vaccinated individuals was sampled approximately 4 to 7 months after the last dose.
The pVNT50 for each group is shown at the top of each graph as the geometric mean titer (GMT), along with the fold reduction relative to the PT strain; the 1 bar represents the 95% confidence interval.
The horizontal dashed line represents the lower limit of detection of the pseudovirus neutralization assay. A pVNT50 value of less than 10 indicates a negative sample, which is counted as 5.
Neutralizing antibody titers against all omicron subtype variants tested were significantly lower than the corresponding titers against PT strains in each vaccinated group, a finding that indicates substantial immune escape for omicron subtype variants. Decreased neutralization titers were associated with spike mutations.
Neutralization of BA.1.1 and BA.2 was similar (within 1.5-fold) to that of BA.1. Neutralization of BA.2.12.1 (which has an additional L452Q mutation in its RBD compared to BA.2) was 1.4-1.7-fold lower.
In each vaccination group, neutralizing antibody titers against BA.4 and BA.5 were 2.1-2.6-fold lower than those against BA.2 subtype variants, while BA.4 and BA.5 were the most The predominant subtype variant in the Americas and possibly the next globally prevalent subtype variant.
This finding suggests that two mutations in the RBD (L452R and F486V) result in lower neutralization efficiency of antibodies induced by current vaccines based on PT sequence design compared to the RBD of the BA.2 subtype variant.
To develop a better ZF2001 vaccination strategy, we collected samples 1 month after recipients received the third dose of the vaccine.
The group was then subdivided into three subgroups based on the interval between the second and third doses: 1 month, 2 months, and 4 to 6 months (long-interval subgroup).
In addition, to examine the persistence of neutralizing antibodies following ZF2001 vaccination in the long-interval subgroup, we collected serum samples 4–7 months after the third dose.
We found that neutralizing antibody titers increased with the interval between the second and third doses, especially against the omicron subtype variant.
Compared with the vaccinates with a 1-month interval between the second and third doses, the neutralizing antibody titers against the PT strain reached nearly 10-fold in the vaccinates with an interval of 4 to 6 months, and all omicron subtypes were titers of neutralizing antibodies.
The neutralizing antibody titer of the mutant strain reached about 30 times (P<0.001).
Long-interval subgroup vaccinators were 100% seropositive for all omicron subtype variants tested. Neutralizing antibody titers and seropositivity rates against all subtype variants in samples collected 6 months after the last dose of the vaccine in the long-interval subgroup were higher than those in the short-interval subgroup after the last dose Samples collected 1 month after the vaccine.
Neutralizing antibody titers against the PT strain and all omicron subtype variants were higher in the heterologous booster group than in the group that received three doses of the same inactivated vaccine.
However, the allogeneic booster group was greater than the inactivated vaccine group in terms of fold reduction in responses against the BA.2, BA.2.12.1, BA.4 and BA.5 subtype variant strains relative to the response against the PT strain.
This finding suggests that these subtype variant strains have more mutations in the RBD, which lead to immune escape.
The rapid emergence of new variants makes the development of variant-specific vaccines difficult.
Our findings suggest that developing better vaccination strategies for existing vaccines may help improve neutralization levels against omicron subtype variants.
Because the ZF2001 vaccine contains protein subunits and the antigens are concentrated on the RBD, the neutralizing antibody titer against the omicron subtype variant can be increased by multiple booster doses and immune maturation after vaccination.
However, in order to better prevent the immune escape of the current subtype variant strains (especially BA.4 and BA.5) and the subtype variant strains that may be circulating in the future, it is still necessary to develop an updated version of the vaccine as a booster shot.
Omicron BA.4/5 escape vaccine has strong ability and the vaccine needs to be optimized
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



