First RSV vaccine: Pfizer and GSK have successively passed FDA committee review
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
First RSV vaccine: Pfizer and GSK have successively passed FDA committee review
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
First RSV vaccine: Pfizer and GSK have successively passed FDA committee review.
Who got the first RSV vaccine? Pfizer and GSK have successively passed FDA committee review, and the final results will be announced in May.
GlaxoSmithKline (GSK)’s respiratory syncytial virus (RSV) vaccine was voted on Wednesday by the U.S. FDA’s Vaccines and Related Biological Products Advisory Committee.
Lower respiratory tract disease due to RSV in adults. In addition, ten consultants agreed with the safety of the vaccine, and two consultants disagreed.
It is worth noting that the committee also conducted an evaluation of Pfizer ‘s RSV vaccine on Tuesday .
In the end, Pfizer’s RSV vaccine won the support of experts with a narrow margin of 7:4, among which the experts who held opposing opinions mainly expressed concerns about the safety of the product.
Based on the voting results of the two meetings, committee experts seem to be more confident in GlaxoSmithKline ‘s vaccine data.
Still, some advisers say the RSV vaccine could raise additional safety concerns once it hits the market, and regulators need to be cautious.
Overall, both GlaxoSmithKline and Pfizer have reached the sprint stage. Who can take the lead in the RSV vaccine field?
At present, the FDA plans to release the final results of the approval of the GlaxoSmithKline vaccine on May 3, and the approval results of the Pfizer vaccine will also be released in May.
Normally, the organization will follow the recommendations of the expert panel.
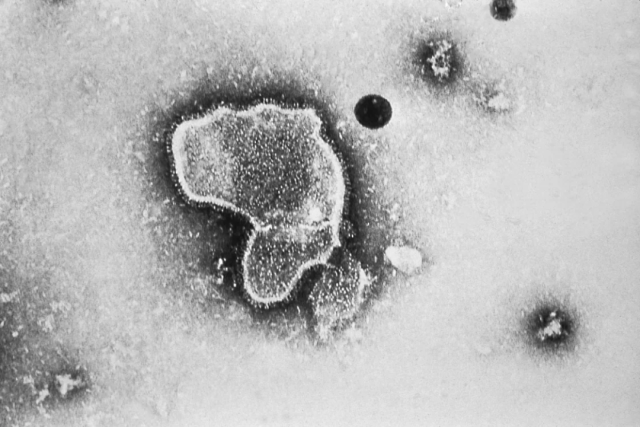
(Source: CDC)
Both RSV vaccines, from Pfizer and GlaxoSmithKline, are for adults 60 and older. Prior to this, RSV killed a large number of older adults each year due to the lack of an available RSV vaccine.
According to the trial data submitted by both parties, the effectiveness of GlaxoSmithKline ‘s RSV vaccine in preventing lower respiratory tract diseases caused by RSV during the trial period was about 82.6%, while the effectiveness of Pfizer’s vaccine in the same population was 66.7%. %.
In addition, possible side effects caused by the RSV vaccine became the focus of the review team.
During Pfizer ‘s RSV vaccine trial, two cases of Guillain-Barré syndrome (GBS) were diagnosed.
In the GlaxoSmithKline trial, there was also a case of GBS possibly related to the vaccine, and compared with the control group, the vaccine recipients also had a higher rate of arrhythmia.
Guillain-Barré syndrome is a rare immune-mediated disease.
Its lesions often occur in the spinal nerves, cranial nerve roots, etc., resulting in symptoms such as muscle weakness, quadriplegia, or cranial nerve paralysis.
However, most people with the disease go into remission and recover with treatment.
Experts from an FDA advisory committee believe that cases of Guillain-Barré syndrome could lead to significant safety concerns.
The fact that such a rare disease showed up in both companies’ trials is troubling. Typically, Guillain-Barré syndrome occurs in about one in 100,000 people aged 60 and over.
In trials, however, this ratio has been stretched to around 1 in 9,000.
After discussion at the meeting, experts in the advisory committee generally believed that the current RSV vaccine needs more safety data to increase its leverage.
And once these RSV vaccines are approved, long-term safety monitoring for them will become “critical.”
At present, the FDA has asked Pfizer to conduct an additional study after its vaccine is approved to evaluate the probability and risk of GBS among vaccine recipients.
GlaxoSmithKline also said it is currently paying close attention to safety issues during the trial and will continue to monitor safety after approval.
References:
1. https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-panel-votes-gsks-rsv-vaccine-2023-03-01/
2. https://www.cidrap.umn.edu/respiratory-syncytial-virus-rsv/close-vote-fda-advisers-recommend-pfizer-rsv-vaccine-those-60-and
Pfizer and GSK have successively passed First RSV vaccine: Pfizer and GSK have successively passed FDA committee review, and the final results will be announced in May.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



