NEJM: This fatal heart disease can be reversed or the first time
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
NEJM: This fatal heart disease can be reversed or the first time
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
NEJM: This fatal heart disease can be reversed or the first time
Transthyretin amyloid cardiomyopathy (ATTR-CM) is an amyloidosis affecting the heart that is a progressive disease resulting from the dissociation of transthyretin into monomers and misfolding into amyloid Deposition in the myocardial interstitium leads to progressive heart failure, with half of patients dying within four years of diagnosis. The disease is also considered irreversible.
However, a recent report published in the New England Journal of Medicine ( NEJM) showed that three men aged 68, 76 and 82 who were diagnosed with ATTR-CM recovered on their own.
Symptoms disappeared, and tests, including cardiovascular magnetic resonance (CMR) scans, showed that amyloid buildup had cleared their hearts.
The report, entitled: Antibody-Associated Reversal of ATTR Amyloidosis–Related Cardiomyopathy , was published in the New England Journal of Medicine (NEJM) on June 8, 2023 , and the authors are mainly from University College London.
This research not only represents a major breakthrough in the understanding of cardiac amyloidosis, but also opens up new possibilities for more effective treatment options.

Professor Marianna Fontana , the first author of the study , said that this is the first time that the hearts of patients with ATTR-CM can actually return to health, which also brings the possibility of developing new treatments.
The research team also found that the three patients developed a specific immune response to amyloid, while amyloid-targeting antibodies were not found in other patients whose disease progressed normally.
Corresponding author Professor Julian Gillmore said it had not yet been conclusively proven whether these antibodies led to the patient’s recovery.
However, existing data suggest that this is highly likely, and that the antibody could be recreated in the lab and used as a treatment, which the research team is currently investigating further.
Transthyretin amyloidosis (ATTR) is caused by amyloid deposits of a blood protein called transthyretin (TTR) , which can be either hereditary or non-inherited. If these proteins accumulate in the heart, they cause transthyretin amyloid cardiomyopathy (ATTR-CM) .
For this type of disease, current treatments aim to alleviate the symptoms of heart failure, but cannot solve the problem of amyloid deposition.
There are some ongoing clinical trials, such as the use of CRISPR-Cas9 gene editing technology to reduce amyloid in the blood. TTR protein levels, thereby slowing the formation of amyloid.
Due to the lack of specific clinical manifestations of ATTR-CM in the early stage, the misdiagnosis rate is high.
In other words, the actual incidence of the disease is higher than we think. Previously, diagnosing the disease required a biopsy of tissue taken from the heart, but now, with advances in imaging technology, we can monitor the disease more precisely, monitor the burden of amyloid on the heart, track the progression of the disease, and identify signs of disease reversal.
The study began when the team discovered that a 68-year-old ATTR-CM patient reported an improvement in his symptoms.
After reviewing the records of 1,663 patients with ATTR-CM, the team found two more ATTR-CM cases with disease reversal.
The disease reversal and recovery of the three male patients was monitored by blood tests, several imaging techniques and one patient was also evaluated for exercise capacity.
The test results showed that their heart structure and function had returned to a near-normal state, and the amyloid protein in the heart was almost completely cleared.
One of the recovered patients underwent a biopsy of the heart muscle, and the team found an atypical inflammatory response (including macrophages) surrounding the amyloid deposits , suggesting an immune response.
No such inflammatory response was found in biopsies from the other 286 patients whose disease progressed normally.
After further investigation, the research team found antibodies in the three patients that could specifically bind to ATTR amyloid deposits in mouse and human tissues, as well as to synthetic ATTR amyloid.
No such antibodies were found in the other 350 patients in this cohort with a typical clinical course.
If these antibodies can be harnessed, they could be combined with currently being tested therapies that inhibit the production of the TTR protein to help patients clear the disease-causing amyloid from their bodies and prevent its further deposition.
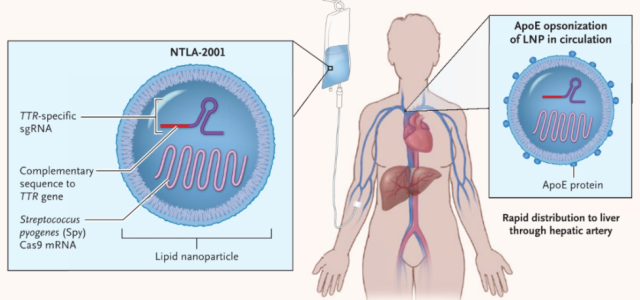
Currently, the most promising treatment for transthyretin amyloidosis (ATTR) is NTLA-2001, a CRISPR-Cas9 gene editing therapy developed by Intellia , a gene editing therapy company founded by Nobel laureate Jennifer Doudna .
In June 2021, the team led by Professor Julian Gillmore published a paper in the New England Journal of Medicine (NEJM) , reporting the effect of NTLA-2001 on the treatment of transthyretin amyloidosis (ATTR) , which is also the first publication in the world Clinical trial results of in vivo CRISPR gene editing therapy.
The therapy performs efficient gene editing on liver cells by direct injection of lipid nanoparticle (LNP) delivery vectors carrying sgRNA targeting the disease-causing gene TTR gene and optimized spCas9 protein mRNA sequence. After treatment, the patient’s serum transthyretin (TTR) level continued to decrease.
Paper link :
https://www.nejm.org/doi/full/10.1056/NEJMc2304584
https://www.nejm.org/doi/full/10.1056/NEJMoa2107454
NEJM: This fatal heart disease can be reversed or the first time
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



