FDA approved a new drug for alopecia areata: Restore 80% of hair growth
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA approved a new drug for alopecia areata: Restore 80% of hair growth
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
FDA approved a new drug for alopecia areata: Restore 80% of hair growth.
It can restore 80% of hair growth. After one year, the FDA approved a new drug for alopecia areata, which can treat adolescent alopecia areata
On June 23, 2013, Pfizer announced that the US FDA approved its LITFULO ™ ( Ritlecitinib ) for the treatment of severe alopecia areata patients over 12 years old.
This is a once-daily covalent JAK3/TEC dual kinase inhibitor with an approved dose of 50mg/time. This is the first and only FDA-approved drug for the treatment of adolescent patients (12 years and older) with severe alopecia areata.
It is worth mentioning that a year ago, in June 2022, the FDA approved the oral JAK inhibitor baricitinib (baricitinib) developed by Eli Lilly and Incyte for the treatment of alopecia areata in adults, which is the first FDA-approved treatment . Systemic therapy for alopecia areata.
Alopecia areata , an autoimmune disease characterized by patchy hair loss, is the second most prevalent form of hair loss worldwide , after androgenetic alopecia.
Alopecia areata is the partial or complete loss of hair on the scalp, face or other parts of the body due to immune cells attacking healthy hair follicles.
Alopecia areata has an average age of onset between the ages of 25 and 35, but it can also affect older adults, children, and adolescents, and can be seen in both sexes and all races.
Alopecia areata is associated with poor health-related quality of life in many patients who may suffer from serious psychological disorders, including depression and anxiety.
On March 26, 2022, the New England Journal of Medicine (NEJM) published a clinical trial paper entitled: Two Phase 3 Trials of Baricitinib for Alopecia Areata .
The paper announced the results of two phase 3 clinical trials for the treatment of severe alopecia areata , which aimed to verify the efficacy and safety of the oral JAK inhibitor Baricitinib developed by Eli Lilly / Incyte in the treatment of severe alopecia areata.
The results showed that in the 36th week after treatment, nearly 40% of patients in the 4mg dose group had their hair loss reduced from an average of more than 85% to less than 20%. That is, nearly 40% of patients recovered 80% of their hair.

A woman with near-total hair loss was treated with 4 mg daily baricitinib for 8 months and her hair was almost completely restored
Prior to this , on August 4, 2021, Pfizer announced that Ritlecitinib, a small-molecule drug developed for the treatment of alopecia areata, had achieved positive top-line results in phase 2b/3 clinical trials.
In this phase 2b/3 clinical trial, both 30mg and 50mg doses of Ritlecitinib met the primary efficacy endpoint of improving scalp hair regrowth. After 6 months of treatment, the proportion of patients with ≤20% scalp hair loss was significantly higher than that of the placebo group.
In this randomized, placebo-controlled, double-blind clinical trial, a total of 718 patients with alopecia areata aged 12 and above participated.
These alopecia areata patients had more than 50% of their scalp hair loss and had symptoms of alopecia areata for at least half a year.
Patients were randomly assigned to four groups, Ritlecitinib 50mg, 30mg, 10mg, and placebo.
The primary endpoint was based on the proportion of patients with ≤20% scalp hair loss at week 24. Both 30mg and 50mg doses of Ritlecitinib achieved the primary efficacy endpoint of improving scalp hair regeneration.
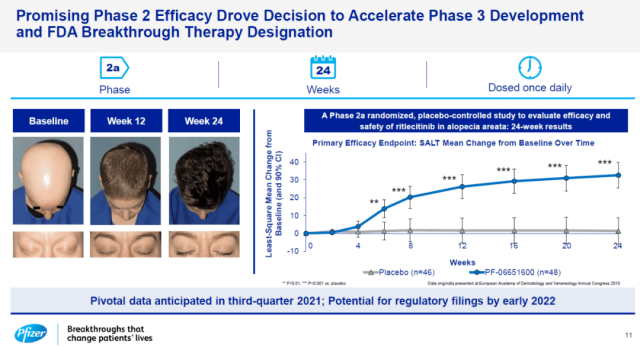
Ritlecitinib , developed by Pfizer, is a JAK3/TEC inhibitor that inhibits IL-15 and CD8 cytokine signaling, two cytokines that drive the immune system to kill hair follicle cells.
Ritlecitini has achieved significant results in previous Phase 2 clinical trials and has been granted FDA Breakthrough Therapy designation.
This innovative therapy has already started clinical research in China. The therapy is also being studied in leukoplakia, rheumatoid arthritis, Crohn’s disease and ulcerative colitis.
References :
https://www.pfizer.com/news/press-release/press-release-detail/fda-approves-pfizers-litfulotm-ritlecitinib-adults-and
https://www.nejm.org/doi/full/10.1056/NEJMoa2110343FDA approved a new drug for alopecia areata: Restore 80% of hair growth
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



