Why are gene-edited babies so risky?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Why are gene-edited babies so risky? Early human embryos fail to effectively repair DNA damage
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Why are gene-edited babies so risky? Early human embryos fail to effectively repair DNA damage, new study finds.
Back in 2015, scientists warned against using CRISPR-Cas9 gene-editing technology to modify the genome of germ cells, fearing that it could have unpredictable consequences.
However, this warning was ignored by Dr. He Jiankui . In November 2018, he announced the birth of ” CRISPR Baby “, which shocked the entire scientific community, and we all know the follow-up.
Beyond the ethical and legal violations of the ” CRISPR Baby ” research, it remains unclear whether such CRSIPR gene editing of germ cells would have negative health effects.
Recently, at the 39th Annual Meeting of the European Society for Human Reproduction and Embryology (ESHRE) , Dr. Nada Kubikova of Oxford University made a report titled: Deficiency of DNA double-strand break repair in human preimplantation embryos revealed by CRISPR-Cas9 , the report pointed out that early human embryonic cells are often unable to effectively repair DNA damage , which has important implications for CRISPR-Cas9 gene editing.
CRISPR-Cas9 gene editing can be used to correct defective genes, a process that requires first breaking the DNA double strand, causing a DNA double strand break, and then repairing the broken DNA double strand.
The new study suggests that applying CRISPR-Cas9 gene-editing technology to human embryos could have unwanted and potentially dangerous consequences.
The report was published in the journal Human Reproduction on June 29, 2023 .
Dr. Nada Kubikova studied the effect of CRISPR-Cas9 gene editing technology on the DNA editing of early embryonic cells.
The results showed that CRISPR-Cas9 gene editing technology can efficiently target the target DNA of embryonic cells.
However, this rarely leads to the expected results. The change that corrects the defective gene more likely results in a permanent break in the double strand of DNA, which can lead to additional heritable abnormal changes in the embryo.
This means that there are significant risks in using CRISPR-Cas9 gene editing in early human embryos.
CRISPR-Cas9-based gene-editing technology has been used in clinical trials to treat adult and pediatric patients with sickle cell disease, genetic diseases such as beta thalassemia, and cancer.
Just on June 8 this year, the FDA accepted the Biologics License Application (BLA) for the CRISPR gene editing therapy exa-cel for the treatment of severe sickle cell disease and transfusion-dependent β-thalassemia, and granted it priority review for the treatment of SCD qualifications.
This is also the first marketing application for a CRISPR gene editing therapy accepted by the FDA.
If it were possible to gene-edit embryos before they were implanted in the womb, more genetic diseases could be avoided and treated, since every cell in the future would be guaranteed to be gene-edited at this point.
However, almost all countries have banned gene editing of human embryos because of the changes that could be passed down in the human genome from generation to generation and because of its uncertain safety.
Dr. Nada Kubikova hopes to further evaluate whether CRISPR-Cas9 can be an effective way to correct genetic errors in human embryos, and to clarify whether this method is safe.
After approval from ethics review, she and her colleagues fertilized donated eggs with donated sperm and created 84 embryos using intracytoplasmic sperm microinjection.
In 33 embryos, they used CRISPR-Cas9 for gene editing to create breaks on the double strand of DNA, and they targeted non-coding DNA regions that did not contain any coding genes to understand how CRISPR-Cas9 affects embryonic cells and its DNA without destroying any genes.
The remaining 51 embryos served as controls.
All cells in our body have efficient mechanisms to repair DNA damage. In most cases, the ends of broken DNA strands are quickly repaired and rejoined. This repair mechanism is important because persistent unrepaired DNA damage prevents cells from functioning properly and can be fatal.
The most common way cells repair DNA is by rejoining the ends of the DNA strands, known as non-homologous end joining (NHEJ) , but this repair method results in the loss or addition of some bases where the DNA strands rejoin.
This repair method may damage the gene. In fact, CRISPR-Cas9 gene editing is to first cause DNA double-strand exercise, and use the error in DNA non-homologous end joining ( NHEJ) repair to achieve the gene knockout effect.
Another way cells repair DNA breaks is to use an intact copy of the damaged region as a template to copy and replace the damaged region, a more precise method known as homology-directed repair (HDR) .
When CRISPR-Cas9 gene editing is used to correct gene mutations, we can provide a DNA sequence (donor sequence) . When cells repair DNA double-strand breaks caused by CRISPR-Cas9, they may use these sequences as templates to repair the original Gene mutation.
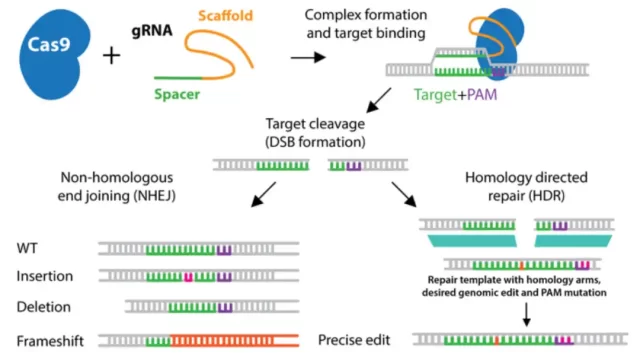 NHEJ restoration (left) and HDR restoration (right)
NHEJ restoration (left) and HDR restoration (right)
In this study, the research team detected changes at the target DNA site in 24 of 25 embryos, suggesting that CRISPR-Cas9 gene editing is very efficient in human embryonic cells.
However, only 9% of target sites were repaired by the homology-directed repair (HDR) process, while 51% of DNA double-strand break sites were subjected to non-homologous end-joining (NHEJ) , generating DNA sequence mutations. The remaining 40% of DNA double-strand breaks cannot be repaired.
Unrepaired breaks in the DNA strands eventually result in the loss or duplication of large segments of chromosomes that extend from the location of the break to the end of the chromosome.
This type of large genomic abnormality can affect the viability of embryos, and if affected embryos are transferred to the uterus and a baby is born, they are at risk of carrying serious congenital abnormalities.
This study shows that homology-directed repair (HDR) is uncommon in early human embryos , and that during the first few days of life, human embryonic cells cannot effectively repair their DNA damage. CRISPR-Cas9 is very efficient at targeting DNA sites.
However, most cells repair DNA double-strand breaks induced by CRISPR-Cas9 using non-homologous end joining (NHEJ) , a process that introduces additional mutations rather than correcting existing ones.
It would be a challenge to try to use CRISPR-Cas9 to correct genetic disorders in human embryos, because most of the time, such attempts are unsuccessful.
Dr. Nada Kubikova said that while the results of this study are a caution against the use of CRISPR-Cas9 genome editing in human embryos, there are some positive findings that suggest that it is possible to modify the way the genome is edited to reduce risk and improve the ability to successfully correct mutations, This holds promise for improving gene editing techniques in the future.
On average, only about a quarter of embryos produced using in vitro fertilization (IVF) make it into a baby. Half of them stopped developing before being transplanted into the womb.
The inability of embryos to effectively repair DNA damage revealed by this study may explain why some IVF embryos fail to develop, a finding that could improve IVF treatments.
Next, the team will look for new ways to protect early embryos from DNA damage, which could lead to potential improvements in fertility treatments, the research team said. They also plan to explore gentler methods of gene editing to avoid DNA double-strand breaks.
Overall, this study further underscores the importance of why gene editing needs to be thoroughly researched and understood before it can be done in human embryos.
In the future, gene editing may become a useful tool for preventing babies from being born with genetic diseases, but we now need time to determine how to use gene editing tools successfully without unanticipated risks.
This also means that we need to strictly regulate related research. This research also helps us understand how to improve fertility treatments.
References :
https://doi.org/10.1093/humrep/dead093.089
https://www.genengnews.com/topics/genome-editing/warnings-to-avoid-using-crispr-cas9-on-human-embryos-reinforced-by-new-findings/
Why are gene-edited babies so risky? Early human embryos fail to effectively repair DNA damage
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



