First CRISPR Gene Editing Therapy Nears FDA Approval
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
First CRISPR Gene Editing Therapy Nears FDA Approval for Sickle Cell Disease Treatment
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
First CRISPR Gene Editing Therapy Nears FDA Approval for Sickle Cell Disease Treatment
Global First CRISPR Gene Editing Therapy Receives Key Endorsement from FDA Committee, Expected Approval Next Month.
Swiss biotechnology company CRISPR Therapeutics saw its stock surge over 20% in pre-market trading on Wednesday, and it’s clear from the company’s name that something good is happening in the gene editing industry.
In simple terms, the company announced that the U.S. Food and Drug Administration’s Cellular, Tissue, and Gene Therapies Advisory Committee has completed the evaluation of CRISPR Therapeutics and Vertex Pharmaceuticals’ gene editing therapy, Exa-cel, for the treatment of severe sickle cell disease (SCD) in patients aged 12 and above who experience recurrent vaso-occlusive crises.

(Source: CRISPR Therapeutics)
Just like in the past few years when vaccine reviews were taking place, a positive recommendation from an external committee does not bind the FDA’s decision on whether to approve the therapy for market release. However, the FDA generally follows the committee’s advice. According to the established review schedule, the FDA will evaluate the eligibility of this drug for treating severe sickle cell disease on December 8th and approve the biologics license for this therapy to treat transfusion-dependent beta-thalassemia (TDT) next year.
For the investment market, the key takeaway here is that the first-ever therapy for treating genetic diseases using CRISPR/Cas9 gene editing technology is expected to receive FDA approval in one month.
By the way, one of the co-founders of CRISPR Therapeutics, Emmanuelle Charpentier, received the Nobel Prize in Chemistry in 2020 for her work in CRISPR gene editing.
Hopes for Potential “Functional Cures”
According to the Merck Manual, sickle cell disease is a hereditary genetic disorder of hemoglobin (the oxygen-carrying protein in red blood cells), characterized by the red blood cells taking on a crescent (sickle) shape and causing chronic anemia due to the abnormal destruction of red blood cells. Because sickle cells are stiff, they are difficult to pass through the body’s smallest blood vessels (capillaries), leading to blood blockages, pain, organ damage, and other consequences.
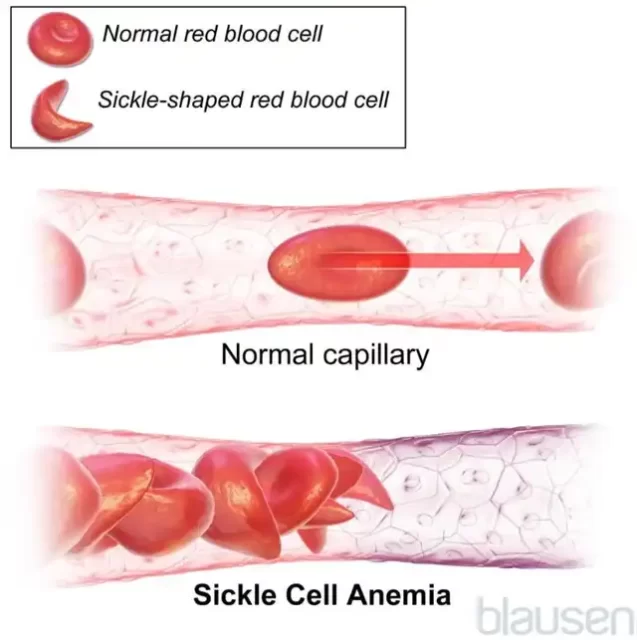 (Illustration, Source: Merck Manual, Blausen)
(Illustration, Source: Merck Manual, Blausen)
Traditionally, the goal of treating this disease has been to alleviate symptoms and prevent crises. While a cure can be achieved through stem cell transplantation, patients need to take immunosuppressive drugs for the rest of their lives.
The Exa-cel therapy works by editing the patient’s own hematopoietic stem cells in vitro to produce high levels of fetal hemoglobin, reducing vaso-occlusive crises in SCD patients. Similarly, this therapy can also reduce the need for blood transfusions in TDT patients. According to previous clinical trial results, most TDT patients who used the Exa-cel therapy no longer needed blood transfusions during the trial period, and all SCD patients in the study did not experience vaso-occlusive crises during the follow-up period.
There’s a Shortcoming on the Application Front
For SCD patients, the FDA will also approve Bluebird Bio’s gene therapy on December 20th. Although there are increasingly more treatment options, they all share a common problem—cost.
While Vertex Pharmaceuticals has not disclosed the price of Exa-cel, it is generally expected that the treatment cost for an individual patient may reach the level of millions of dollars. For reference, economic studies focusing on the U.S. healthcare system estimated that the lifetime treatment of SCD patients in the United States is also very expensive, costing the healthcare system $3 billion annually. At the same time, the costs of other similar gene therapies can also reach several million dollars.
Vertex expects that about 20,000 patients may be eligible for this therapy once it is approved for market release. Federal health assistance and private insurers have also shown interest in including this drug.
Due to the customized nature of “gene editing,” each patient requires individual treatment, which objectively limits the large-scale application of this type of therapy.
As explained, patients initially need to undergo treatment in which bone marrow stem cells are released into the bloodstream, followed by continuous blood transfusions for eight weeks. Afterward, the treated cells are removed and sent to the company for processing. Simultaneously, patients will undergo “intense chemotherapy” to clear the treatment cells from the bone marrow. Finally, the processed cells are infused back into the patient’s body, and they must remain in the hospital for at least a month to ensure that the new cells grow and refill the bone marrow, completing the entire course of treatment.
One of the advisors to Vertex Pharmaceuticals, Dr. Alexis Thompson, the Chief of Hematology at Children’s Hospital of Philadelphia, bluntly stated that the vast majority of U.S. hospitals cannot provide this treatment.
As a novel therapy, the industry also has additional concerns about CRISPR gene editing technology: if the gene “scissors” make a mistake, it could disrupt genes and lead to blood cancer. While this has not occurred in clinical trials, CRISPR’s trials included only 44 patients, and only 30 were followed for over 16 months. To address this issue, the company plans to compare the DNA of enrolled patients with a large database and intends to track the changes in clinical trial participants for the next 15 years. The expert panel acknowledges the therapy’s safety and emphasizes that the benefits of the new therapy outweigh the potential drawbacks.
First CRISPR Gene Editing Therapy Nears FDA Approval for Sickle Cell Disease Treatment
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



