Milestone: FDA approves first human clinical trial of LNP-delivered in vivo CRISPR gene editing
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Milestone: FDA approves first human clinical trial of LNP-delivered in vivo CRISPR gene editing
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Milestone: FDA approves first human clinical trial of LNP-delivered in vivo CRISPR gene editing
The gene editing technology represented by CRISPR-Cas9 has greatly accelerated the development of gene therapy and brought great hope to many difficult diseases that had no cure.
On March 2, 2023, Intellia Therapeutics announced that the FDA approved its investigational new drug (IND) application for NTLA-2002, an in vivo CRISPR therapy for the treatment of hereditary angioedema (HAE) . This is also the first LNP-delivered in vivo CRISPR gene-editing therapy to receive FDA approval for human clinical trials.
NTLA-2002 , an in vivo CRISPR gene editing candidate therapy, delivers the CRISPR-Cas9 gene editing system in the form of mRNA through lipid nanoparticles (LNPs) , targeting knockout of the KLKB1 gene to permanently reduce plasma kallikrein activity, thereby preventing the onset of hereditary angioedema (HAE) .

Intellia Therapeutics is a gene editing therapy company foundedJennifer Doudna, the founder of CRISPR gene editing technology andNobel laureate
On June 26, 2021, Intellia published a paper in the top international medical journal ” New England Journal of Medicine ” (NEJM) , reporting the effect of CRISPR gene editing therapy NTLA-2001 in the treatment of transthyretin amyloidosis (ATTR) .
It is the world’s first published clinical trial results of in vivo CRISPR gene editing therapy.
This clinical study has greatly expanded the scope of application of CRISPR gene editing therapy.
Direct injection of CRISPR gene editing components delivered by LNP can perform efficient gene editing in vivo, which opens up a new way for the treatment of many genetic diseases. ” Opening a New Era in Medicine “.
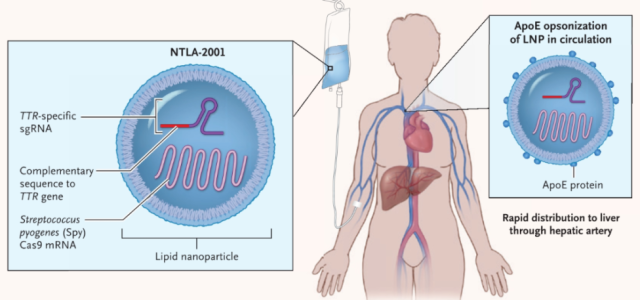
In June 2022, Intellia announced the results of the mid-term clinical trial of NTLA-2001 therapy.
he results showed that during the follow-up period of 2 months to 12 months after treatment, the serum transthyretin (TTR ) level of patients continued to decline. This also illustrates that LNP-delivered CRISPR gene editing can produce long-term therapeutic effects in vivo.
NTLA-2002 , the second in vivo CRISPR gene-editing therapy developed by Intellia, is designed to evaluate the safety, tolerability, and efficacy of NTLA-2002 in adult patients with type I or type II hereditary angioedema (HAE). Kinetics and pharmacodynamics, including determination of the level of kallikrein activity in plasma after treatment and the incidence of HAE.
Among them, the phase 1 clinical trial is an open-label, single-dose escalation design, which is used to determine different dose levels of NTLA-2002, and the phase 2 clinical trial will conduct randomized, placebo-controlled further evaluation.
Hereditary angioedema (HAE) is a rare genetic disorder affecting approximately 1 in 50,000 people. It is characterized by severe, recurring, and unpredictable episodes of inflammation in various organs and tissues of the body that can be painful, debilitating, and even life-threatening.
Current treatment options are typically lifelong, requiring chronic intravenous or subcutaneous administration twice weekly, or daily oral administration.
But even with long-term medication, breakthrough attacks can still occur. Inhibition of kallikrein ( kallikrein ) is a clinically proven strategy to prevent hereditary angioedema (HAE) .
The therapeutic principle of NTLA-2002 is to deliver CRISPR-Cas9 gene editing through LNP to target the KLKB1 gene of liver cells, thereby inhibiting the production of kallikrein ( kallikrein ) , thereby inhibiting the production of bradykinin (bradykinin) , and bradykinin Overproduction of α can lead to the onset of hereditary angioedema (HAE) .
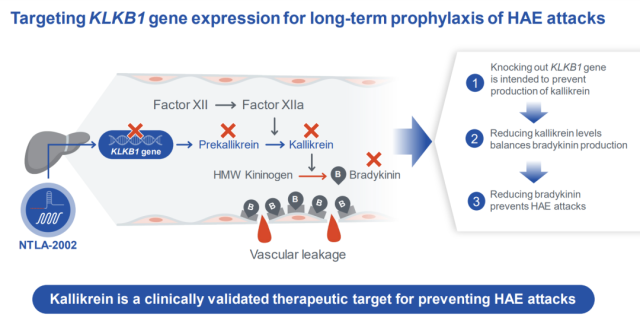
On November 12, 2022, Intellia announced the interim data of the Phase 1/2 clinical trial of NTLA-2002 therapy in the treatment of hereditary angioedema (HAE) .
These clinical data from 10 adult patients with hereditary angioedema (HAE) who received different doses of NTLA-2002 therapy showed that all patients treated with different doses (25mg, 50mg, 75mg) All decreased significantly, 64%, 81%, and 92% at the 32nd week, 22nd day, and 16th week, respectively.
In addition, these data also showed that the frequency of hereditary angioedema (HAE) attacks decreased significantly after a single treatment , with the 25mg and 75mg dose groups reducing the attack frequency by an average of 91% from the first week to the 16th week after treatment. and 78% (when the 50mg dose group had not yet reached the 16th week of observation time) .
At the time of publication of the data, the first three patients treated had been recurrence-free for 5.5-10.6 months. NTLA-2002 was generally well tolerated across all dose groups, with most adverse events being mild.
These clinical data show that NTLA-2002 has the potential of sustained remission after one treatment.
“The FDA’s acceptance of the IND application for NTLA-2002 to initiate clinical evaluation brings us closer to bringing a disruption to patients with hereditary angioedema (HAE) that could change the current treatment paradigm,” said John Leonard , Ph.D. , President and CEO of Intellia. sex therapy.
The approval of the IND for NTLA-2002 marks an important milestone for Intellia, continuing Intellia’s position as a leader in the field of genome editing.
We are excited to advance the development of the NTLA-2002 therapy in the United States, and Intellia is currently working to rapidly enroll patients in a Phase 2 clinical trial and looks forward to publishing additional data from the first-in-human trials of this study later this year.
Intellia R&D pipeline
Intellia’s R&D pipeline is divided into two categories : in vivo CRISPR therapy and in vitro CRISPR therapy .
Two of the in vivo CRISPR therapies are currently in clinical trials, NTLA-2001 (for the treatment of transthyretin amyloidosis) and NTLA-2002 (for the treatment of hereditary angioedema) .
In addition, NTLA-2003 (for the treatment of α1-antitrypsin deficiency liver disease) , NTLA-3001 (for the treatment of α1-antitrypsin deficiency lung disease) and a therapy for the treatment of hemophilia B are under IND application, and the first one is Gene knockout type, the latter two are gene insertion type.
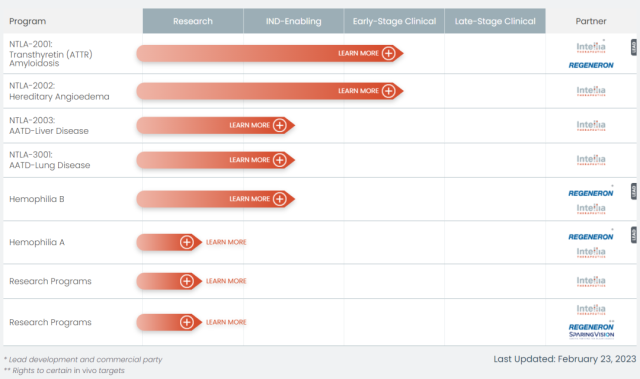
In the in vitro CRISPR therapy, the sickle cell disease therapy developed in cooperation with Novartis, and the independently developed NTLA-5001 for the treatment of acute myeloid leukemia, have previously started clinical trials, but have been deleted from the latest published research and development pipeline.
There is also a general-purpose CAR-T therapy NTLA-6001 for the treatment of CD30-positive lymphoma, which is under IND application.

Soruce reference :
https://www.intelliatx.com/
Milestone: FDA approves first human clinical trial of LNP-delivered in vivo CRISPR gene editing
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



