FDA approved the 1st trial of CRISPR gene editing to treat AIDS in humans
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
FDA approved the 1st trial of CRISPR gene editing to treat AIDS in humans
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
The dawn of a complete cure for AIDS: FDA approved the 1st trial of CRISPR gene editing to treat AIDS in humans.
AIDS, the full name is Acquired Immune Deficiency Syndrome, which is a very harmful infectious disease caused by the human immunodeficiency virus (HIV).
The HIV virus can attack and severely damage the human immune system.
Therefore, AIDS patients often suffer from secondary infections or tumors due to insufficient immunity, which also makes the mortality rate of AIDS patients extremely high.
According to UNAIDS data, the number of HIV carriers and AIDS patients worldwide has increased from 34.3 million at the end of 2013 to 38 million at the end of 2018, and the number is still growing rapidly.
Although the continuous advancement of antiretroviral therapy (ART) has greatly extended the lifespan and prognosis of AIDS patients, it will also bring serious side effects and the emergence of drug resistance.
More importantly, as a retrovirus, HIV can integrate its genome into the chromosome of the host cell. This means that current antiretroviral therapy can only suppress the virus and cannot completely eliminate the HIV virus in the body. There will be no cure for AIDS.

Recently, Excision BioTherapeutics announced that its therapy EBT-101 based on CRISPR gene editing technology has been approved by the US FDA and will begin human clinical trials for the treatment of AIDS.
Excision said that the existing standard treatment for AIDS is antiretroviral therapy, which can prevent the replication of HIV virus in the body, but cannot eliminate HIV virus.
Patients need to receive treatment for a long time, which can cause serious side effects and affect patients. Quality of Life.
The therapy based on CRISPR gene editing technology is expected to replace the current antiretroviral therapy and achieve a “complete cure” for AIDS.
In November 2020, researchers from Temple University and the University of Nebraska Medical Center published research papers in the journal Nature Communications.
Successfully edited and eliminated the SIV-a virus closely related to HIV in the genomes of non-human primates, which is an important step in AIDS research.
This breakthrough also makes mankind closer than ever to the development of a complete cure for AIDS.

This research breakthrough is very close to clinical research, which means that mankind has found a new method that is expected to completely end the AIDS nightmare.
Excision has been authorized by Temple University to conduct clinical research and development. Today, the FDA has also approved its human clinical trials.

As we all know, CRISPR-Cas9 gene editing can accurately cut DNA double-strands, resulting in DNA double-strand breaks.
Based on this principle, we can use CRISPR-Cas9 to cut the HIV virus genome that has been integrated into the human chromosome.
However, if only one site is cut, they are easy to repair, and it is easy to cause genetic mutation near the cut site.
This therapy uses multiple cuttings to completely smash the HIV virus genome, thereby achieving complete elimination of the HIV virus.
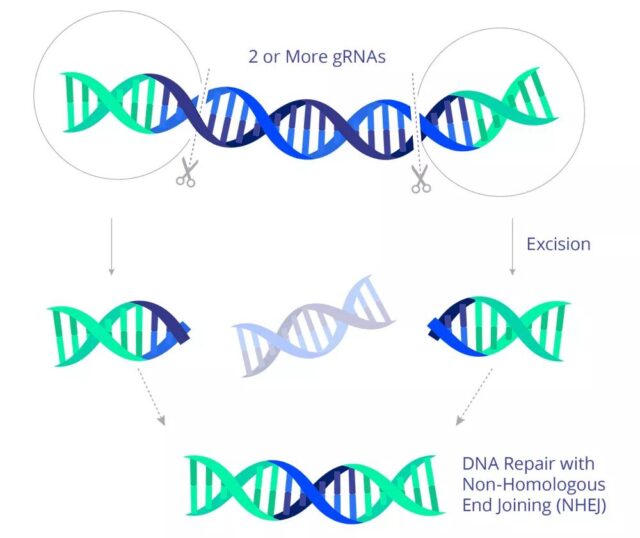
Previous tests in non-human primates have shown that the CRISPR-Cas9 system delivered using adeno-associated virus (AAV9) vectors can reach a wide range of tissues where the bone marrow, lymph nodes, spleen and other viruses are located, especially the important host of the HIV virus. Cell CD4+ T cells, and then eliminate the virus.
It is understood that Excision will officially launch Phase 1/2 clinical trials later this year.
Excision is committed to curing viral infections through CRISPR gene editing technology. Its CRISPR technology is licensed from Nobel Prize winner Jennifer Doudna.
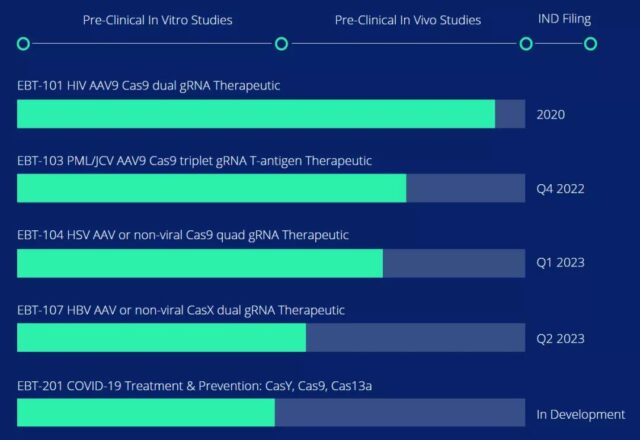
In addition to CRISPR-based AIDS therapy, the company’s R&D pipeline also includes the use of CRISPR technology to eliminate hepatitis B virus (HBV), herpes simplex virus (HSV), papillary polyoma vacuole virus (JCV), and the current outbreak of the COVID-19 Virus (SARS-CoV-2).
Excision’s R&D pipeline
In addition, Moderna, a vaccine development company that has exploded due to mRNA vaccines, also recently announced that it has developed an mRNA-based experimental AIDS vaccine that has been proven to trigger the production of neutralizing antibodies against HIV-like viruses in monkeys.
It is reported that Moderna’s method is different from previous AIDS vaccines. It prevents HIV virus from entering cells by producing broadly neutralizing antibodies against the virus.
This vaccine based on mRNA technology also brings new hope to the repeatedly failed AIDS vaccine research.

Reference:
https://www.excision.bio/technology
https://www.nature.com/articles/s41467-020-19821-7
https://www.fiercebiotech.com/biotech/excision-hiv-crispr-gene-editing-therapy-cleared-for-human-studies-by-fda
https://www.modernatx.com/pipeline/therapeutic-areas/mrna-therapeutic-areas-infectious-diseases
FDA approved the 1st trial of CRISPR gene editing to treat AIDS in humans.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



