UK Becomes the First Country Globally to Approve CRISPR Gene Editing Therapy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
UK Becomes the First Country Globally to Approve CRISPR Gene Editing Therapy
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
UK Becomes the First Country Globally to Approve CRISPR Gene Editing Therapy
On November 16, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) announced on its official website the approval of the CRISPR gene editing therapy Casgevy (abbreviated as exa-cel), developed in collaboration between Forte Pharma and CRISPR Therapeutics.
The therapy is designed for patients aged 12 and above experiencing recurrent vascular occlusive crises associated with sickle cell disease (SCD) and transfusion-dependent beta-thalassemia (TDT).
(Source: MHRA)
The UK regulatory authority specifically emphasized that this marks the world’s first approved gene editing therapy for treating these indications and the first globally approved application of the “gene scissors” CRISPR. This milestone signifies the transformation of this groundbreaking technology, introduced to the world in 2012, into a tangible therapeutic option just 11 years later.
It’s worth noting that Emmanuelle Charpentier, one of the co-founders of CRISPR Therapeutics, was awarded the Nobel Prize in Chemistry in 2020 for her contributions to this revolutionary technology.

(Source: Nobel Prize Official Website)
In response to this news, CRISPR Therapeutics’ stock rose over 5% in pre-market trading, and Forte Pharma, with a market value of $90 billion, experienced a modest increase of 0.6%.
What Sets This Medication Apart?
Due to the conceptual appeal of CRISPR, this medication has been closely monitored by the capital market. According to reports from early November by CNBC, the US Food and Drug Administration’s external advisory committee has also given the green light to the CRISPR gene editing therapies developed by these two companies. As per the schedule, the FDA will conduct a review for the SCD indication on December 8, and a review for the TDT indication will take place next year.
According to the Merck Manual, sickle cell disease is a hereditary genetic abnormality of hemoglobin, characterized by crescent-shaped (sickle) red blood cells. The rigid sickle cells are difficult to pass through the smallest blood vessels (capillaries), leading to blood flow blockages and resulting in pain, organ damage, and other consequences.
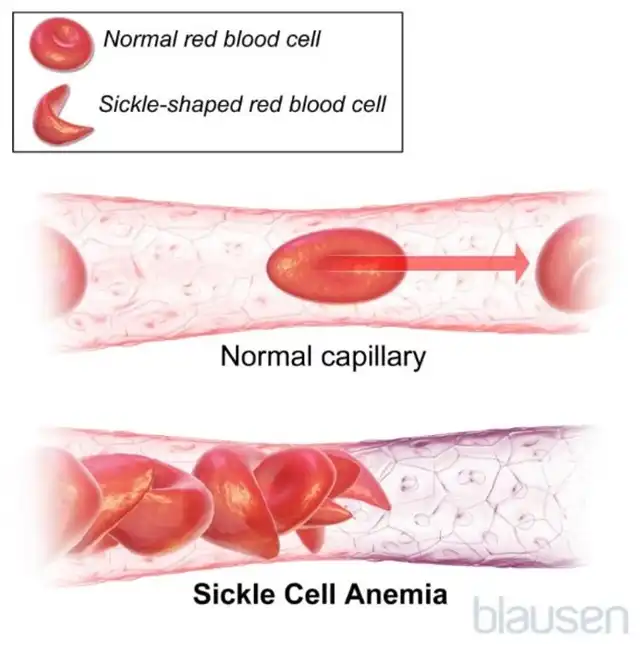
(Illustration, Source: Merck Manual, Blausen)
Traditionally, for such genetic mutation diseases, a cure could only be achieved through bone marrow transplants with lifelong immunosuppressive drug use required for the remaining life of the patient.
The logic of CRISPR gene editing therapy is straightforward: use the patient’s own hematopoietic stem cells for gene editing, and then transfuse them back to the patient, reducing vascular occlusive crises in SCD patients.
In clinical trials involving SCD, 45 patients underwent treatment with the Casgevy therapy, with 29 individuals meeting the criteria for the primary efficacy mid-term assessment after at least 12 months of treatment. Among these 29 individuals, 28 did not experience severe pain conditions within the first 12 months of treatment.
MHRA revealed that there are approximately 15,000 SCD patients in the UK alone. CRISPR Therapeutics also stated in its announcement that, based on the approval conditions set by UK regulators, around 2,000 UK patients qualify for Casgevy.
Although the pharmaceutical companies have not disclosed the pricing, it is speculated that, based on the general pricing of gene therapies, the cost is likely to be in the “million-dollar” range.
Julian Beach, interim executive director of healthcare quality and access at the MHRA, said in the announcement that SCD and TDT are both painful, life-long conditions that, in some cases, can be fatal. Until now, bone marrow transplantation (which must be from a closely matched donor and carries the risk of rejection) is the only permanent treatment option.
Therefore, UK regulators are pleased to announce the approval of the innovative gene-edited treatment Casgevy, which has shown in trials the ability to produce healthy hemoglobin in most patients with SCD and TDT, thus relieving their symptoms.
UK Becomes the First Country Globally to Approve CRISPR Gene Editing Therapy
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



