Daily High-Potency Pill Divarasib Shows 62.5% Effective Response in Treating Resistant Cancers
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Daily High-Potency Pill Divarasib Shows 62.5% Effective Response in Treating Resistant Cancers
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Daily High-Potency Pill Divarasib Shows 62.5% Effective Response in Treating Resistant Cancers
Described as a “game-changer in cancer treatment,” the once-a-day high-potency pill divarasib continues to make a profound impact in the Phase 1b trial, surpassing existing therapies and previous trial results.
Following an independent clinical trial earlier this year, divarasib has now demonstrated a 62.5% effective response when used in conjunction with the existing targeted immunotherapy drug cetuximab for patients with late-stage or metastatic colorectal cancer (CRC) associated with the KRAS G12C gene mutation.
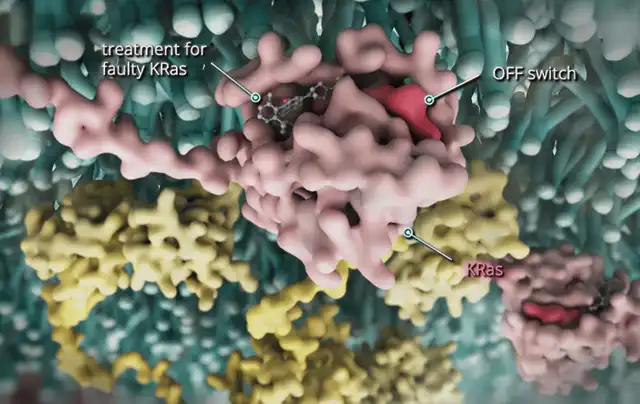
image source:Nature Medicine
In the inaugural trial conducted at the Peter MacCallum Cancer Center in Australia, CRC patients treated solely with divarasib showed a promising 35.9% response rate.
KRAS is a crucial protein regulating cancer cell behavior. For cancer patients with the KRAS G12C gene mutation, their cancer cells are more prone to uncontrolled division and tumor formation, making treatment with existing drugs challenging. Despite only around 4% of cancer patients being affected by the KRAS G12C gene mutation, their prognosis is bleak.
The latest research led by Professor Jeyesh Desai at the Peter MacCallum Cancer Center indicates that when used in conjunction with cetuximab, divarasib can target this mutation, effectively slowing tumor progression with good tolerance and minimal adverse effects.
Desai stated, “The median progression-free survival in patients in the study is slightly over 8 months, with good treatment tolerance and manageable side effects. While not a head-to-head trial, its response rate is superior to other treatment approaches targeting the KRAS G12C mutation that we have seen. We are hopeful that the combination therapy of divarasib and cetuximab will bring better treatment outcomes for our colorectal cancer patients.”
While the KRAS G12C mutation may have the closest association with colorectal cancer, it also plays a crucial role in the accelerated progression of other cancers, such as non-small cell lung cancer (detectable in approximately 13% of patients).
Current treatment for KRAS G12C-positive CRC patients includes 5-FU-based irinotecan, oxaliplatin, and/or capecitabine chemotherapy. However, due to low specificity and high toxicity for specific tumors, this treatment approach faces limitations.
Earlier this year, the cancer center initiated a global Phase I trial for divarasib treatment involving 137 cancer patients. The study found that the drug’s specificity increased 50-fold, and efficacy improved 20-fold compared to other similar drugs used to treat KRAS mutations.
Desai stated in August, “We spent years researching to gain a deeper understanding of how to target KRAS mutations and refine the science to develop molecules with stronger efficacy. This once-a-day pill treatment is a true form of precision medicine, specifically targeting gene mutations driving cancer.”
The research was published in the journal “Nature Medicine.”
Daily High-Potency Pill Divarasib Shows 62.5% Effective Response in Treating Resistant Cancers
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



