Decoding the Impact of Immune Inhibitory Cytokines in Tumor Immunotherapy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Decoding the Impact of Immune Inhibitory Cytokines in Tumor Immunotherapy
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Decoding the Impact of Immune Inhibitory Cytokines in Tumor Immunotherapy.
Changes in the Tumor Microenvironment Due to Immune Inhibitory Cytokines.
Alterations in the epigenetics and growth dynamics of cancer cells lead to the secretion of various cytokines that control the activity of immune cells, promoting an environment conducive to tumor growth.
Among these, the secretion of immune-regulatory cytokines plays a crucial role in recruiting immune inhibitory cells. In the tumor microenvironment, these cytokines recruit other immune inhibitory cells, responsible for phenotype and function shifts in effector immune cells, supporting tumor development.
Phenotypic transformation of immune cells further increases the levels of immune inhibitory cytokines, rendering the tumor environment resistant to the activity of anti-tumor immune cells. Major sources of regulatory cytokines include MDSCs, TAMs, Treg, and Breg cells.
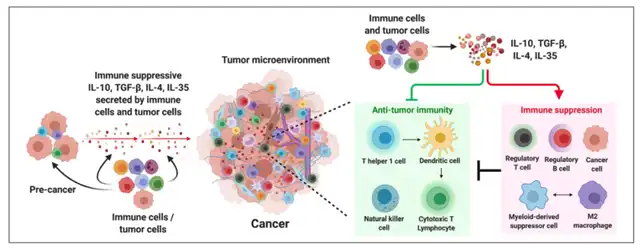
In a broad array of immune inhibitory cytokines, IL-10, TGF-β, IL-4, and IL-35 dominate in the tumor environment across various cancers, primarily responsible for immune suppression.
These cytokines alter the fate of innate and adaptive immune cells, regulating the growth and proliferative capacity of cancer cells.
Simultaneously, immune inhibitory cytokines modify the epigenome and transcription networks of naive immune cells, favoring the formation of the immune inhibitory cell lineage.
Moreover, these cytokines can directly act on cytotoxic CD8+ T cells or natural killer (NK) cells, inhibiting their effector functions and proliferation.
IL-10
IL-10, a multifunctional immune inhibitory cytokine, regulates immune responses and vascular development. Secreted by cancer cells and various immune cells, including myeloid and lymphoid cells, IL-10 exhibits dual functions in promoting and inhibiting tumors. On one hand, IL-10 inhibits cytotoxic T cell activation by downregulating MHC expression on cancer cells and APCs, preventing antigen-specific T cell recognition.
On the other hand, high doses of IL-10 enhance the proliferation and cytotoxic activity of CD8+ T cells.
IL-10 can suppress inflammatory states that may promote tissue damage and tumor occurrence, preventing subsequent tumor development.
Recent research indicates that IL-10 fusion protein based on siltuximab (CmAb-IL10) demonstrates robust anti-tumor effects by blocking dendritic cell-mediated apoptosis of infiltrating CD8+ T cells in tumors.
Combining CmAb-IL10 with immune checkpoint inhibitors significantly improves anti-tumor immunity in late-stage tumor mice.
Therefore, it is necessary to assess potential therapeutic interventions by inhibiting or promoting the IL-10 pathway based on specific cancer types and patient subgroups.
TGF-β
As an immune inhibitory cytokine, TGF-β exerts broad inhibitory effects on immune responses through various mechanisms. TGF-β reduces the differentiation and function of Th1, Th2 cells, and cytotoxic T lymphocytes (CTLs), all of which are crucial for anti-tumor responses. Additionally, TGF-β enhances immune tolerance and tumor evasion by regulating the number and function of Treg cells. TGF-β modulates the fate of immune cells by inhibiting or stimulating cell proliferation, influencing the development of thymic and peripheral T cells.
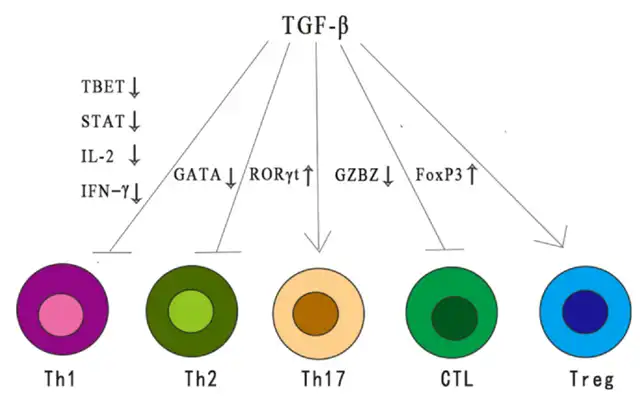
TGF-β plays a dual role in the tumor microenvironment (TME), influenced by different stages of tumor development and genetic background changes. In early tumors, the TGF-β pathway induces cell apoptosis and inhibits cell proliferation, including cancer cells. Paradoxically, in later stages, it promotes tumor progression through regulation of genomic instability, EMT, angiogenesis, immune evasion, and metastasis. When the pro-tumor functions outweigh the anti-tumor effects of TGF-β signaling in cells, it consistently acts as a tumor promoter.
Despite the complexity of TGF-β signal transduction, targeted TGF-β signaling drugs have shown potent activity in cancer treatment. These drugs, including small molecules targeting TGF-β receptors, antisense oligonucleotides, vaccines, neutralizing antibodies, and receptor IgG-Fc fusion proteins, exhibit strong activity. Galunsertib (LY2157299), the first small molecule inhibitor to enter clinical trials, inhibits TGF-βRI kinase. In a phase 1/2 clinical trial, combining Galunisertib with gemcitabine significantly improved overall survival in patients with pancreatic adenocarcinoma compared to placebo plus gemcitabine. TEW-7197 is another TGF-βRI kinase inhibitor.
GC-1008 (fresolimumab), a human monoclonal antibody neutralizing all subtypes of TGF-β, and M7824 (Bintrafusp-alfa), a bifunctional molecule composed of an anti-PD-L1 antibody and the extracellular domain of TGF-βRII, which “captures” TGF-β in the TME, demonstrate therapeutic potential. ABBV-151, influencing the complex process of active TGF-β release, is another therapeutic intervention. The anti-GARP antibody ABBV-151 significantly slows lung metastasis in breast cancer cell lines by blocking its binding with LAP.
Belagenpumatucel-L (Lucanix), a therapeutic, genetically modified allogeneic tumor cell vaccine for NSCLC, inhibits TGF-β2 mRNA production. Although a phase 3 trial for NSCLC did not meet its predefined clinical endpoints, additional analysis showed encouraging results in subgroups. In patients randomized to receive Lucanix after first-line chemotherapy within 12 weeks, a significant increase in overall survival was observed. Trabedersen (ap12009), an antisense oligonucleotide targeting the TGF-β2 gene mRNA region, showed inhibitory effects by downregulating TGF-β2 mRNA in a phase 2 study, resulting in a higher 2-year survival rate for recurrent/refractory advanced glioma patients compared to standard chemotherapy.
IL-4
IL-4 primarily influences lineage-specific differentiation of Th2 cells and regulates humoral immune responses. Secreted mainly by Th2 cells through autocrine signaling, IL-4 plays diverse roles in the tumor microenvironment, exhibiting both immune inhibitory and anti-tumor activities.
Studies indicate that blocking IL-4 can reduce the generation of immune inhibitory M2 phenotypes and MDSCs in specific tumor microenvironments, improving tumor-specific CD8+ T cell responses in mouse models of colon and breast cancer. Additionally, blocking IL-4 can enhance the responsiveness of anti-OX40 immunotherapy. Similar studies in a mouse model of non-small cell lung cancer suggest that blocking IL-4 increases IL-12 production by tumor antigen-specific dendritic cells, enhancing infiltration and proliferation of effector T cells, and reducing tumor burden. Moreover, in a mouse model of anaplastic thyroid cancer (ATC), IL-4 deficiency enhances anti-tumor immunity and overall survival without apparent toxicity.
Research suggests that IL-4 is a novel therapeutic target; blocking it can reduce tumor-induced immune suppression, improving the efficacy of conventional cancer treatments. Therefore, similar to other immune inhibitory cytokines, IL-4 is a key regulatory factor in tumor development and metastasis. It presents an attractive immunotherapy target, either alone or in combination with current effective cancer treatment regimens.
IL-35
Compared to IL-10, TGF-β, and IL-4, IL-35 is a relatively newly discovered heterodimeric cytokine belonging to the IL-12 family. Comprising p35 and EBi3 subunits, IL-35 plays a crucial role in the development of both benign and malignant tumors, including hepatocellular carcinoma, advanced breast cancer, pancreatic ductal adenocarcinoma, non-small cell lung cancer, and prostate cancer. While earlier studies indicated that IL-35 is mainly produced by Tregs, recent evidence confirms its expression in tumor cells.
In vivo, IL-35 promotes tumor growth, progression, and metastasis by enhancing the secretion of other cytokines, such as IL-6 and G-CSF (granulocyte colony-stimulating factor). IL-35 also inhibits various cytokines, including IFN-γ, to achieve pro-tumor effects. Accumulated data suggests that IL-35 participates in the interaction between malignant tumor cells and surrounding immune cells in the tumor microenvironment, inducing an immune inhibitory environment and restricting the involvement of effective anti-tumor immune responses. With the discovery and isolation of various IL-35+ immune cell types like M1 TAMs and DCs, current evidence suggests that tumor-derived IL-35 broadly contributes to the pro-tumor characteristics of different cellular environments. It likely does so by inhibiting the infiltration of tumor-infiltrating lymphocytes (TILs) and the proliferation of effector cells. Overall, IL-35 produced by malignant tumor cells and surrounding stromal cells contributes to immune suppression in the tumor microenvironment, supporting sustained tumor growth and metastasis.
Conclusion
Cytokines play a crucial role in determining the fate of tumor development in the tumor immune microenvironment.
These immune inhibitory cytokines restrict the proliferation and effector functions of anti-tumor immune cells, alter their plasticity, and transform them into inhibitory phenotypes.
Due to restricted infiltration or a lack of effector immune cells, immune checkpoint inhibitors fail to exert optimal effects.
Therefore, targeting or depleting these cytokines has significant clinical benefits. Several cytokines play immune inhibitory roles in tumor development, depending on the tumor type and tumor immune environment.
Recent studies highlight the crucial roles of IL-10, TGF-β, IL-4, and IL-35 in limiting anti-tumor immunity and the progression of tumor growth.
Blocking immune inhibitory cytokines like IL-10, TGF-β, IL-4, and IL-35 may have significant effects in sensitizing tumors to conventional and immune therapies, activating anti-tumor immunity, and controlling tumor development.
Decoding the Impact of Immune Inhibitory Cytokines in Tumor Immunotherapy
References:
Tumor-promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022; 10: 20503121211069012.
Roles of TGF-β signaling pathway in tumor microenvironment and cancer therapy. Int Immunopharmacol. 2020 Oct 21;89(Pt B):107101.
IL-35 Regulates the Function of Immune Cells in Tumor Microenvironment. Front Immunol. 2021;12: 683332.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



