Autophagy at the forefront of tumor immunotherapy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Autophagy at the forefront of tumor immunotherapy
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Autophagy at the forefront of tumor immunotherapy.
Autophagy is a regulatory mechanism that removes unnecessary or dysfunctional cellular components and recycles metabolic substrates.
In response to stress signals in the tumor microenvironment, autophagy pathways are altered in tumor cells and immune cells, resulting in differential effects on tumor progression, immunity, and therapy.
Recent studies have shown that the autophagy pathway is involved in the survival and apoptosis, differentiation, activation, effector function, and trafficking of immune cell subsets to tumors.
At the same time, tumor autophagy can alter tumor growth by modulating immune responses.
Furthermore, the tumor-promoting effect of autophagy can be abolished by combining immune checkpoint therapy with autophagy inhibitors.
Therefore, autophagy is a complex but promising target in cancer therapy.
Autophagy of tumor cells
The tumor microenvironment ( TME ), plays a key role in cancer progression, metastasis and therapy resistance.
In the TME, autophagy in tumor cells can be induced by intracellular and extracellular stress signals, including metabolic stress, hypoxia, redox stress, and immune signals.
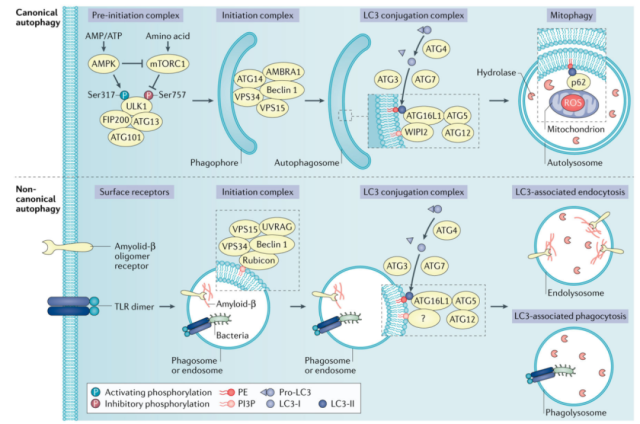
Metabolic stress
Insufficient nutrient uptake by the TME affects metabolic mechanisms, leading to intracellular metabolic stress.
In response to metabolic stress, tumor cells reprogram their own metabolic pathways by upregulating nutrient transporters and activating autophagy.
Mechanistically, 5′-AMP-activated protein kinase ( AMPK ) and mTOR complex 1 ( mTORC1 ) are two opposing regulatory kinases that alter autophagy induction under nutrient deprivation.
As an AMP sensor, AMPK is activated by an increase in the ratio of AMP and ATP, whereas the activity of mTORC1 is decreased by amino acid deprivation.
This results in the phosphorylation of targets in the pre-autophagy initiation complex, thereby initiating autophagy.
Hypoxic stress
Hypoxia, a hallmark of solid tumors, leads to inhibition of mitochondrial oxidative phosphorylation, increased AMP to ATP ratio, and activation of AMPK, while hypoxia also inhibits mTOR signaling.
In addition, hypoxia leads to the activation of activating transcription factor 4 ( ATF4 ), which upregulates the expression of LC3B and autophagy-related protein 5 ( ATG5 ), and maintains high levels of autophagy.
Oxidative stress
Oxidative stress reflects an imbalance between free radicals and antioxidants.
Reactive oxygen species ( ROS ) are generated intracellularly through oxygen metabolism and other processes, and excess ROS may increase the risk of DNA damage and promote tumorigenesis.
In response to elevated ROS, ataxia telangiectasia mutated ( ATM ) activates the TSC2 tumor suppressor through liver kinase B1 ( LKB1 ) and AMPK metabolic pathways in the cytoplasm to inhibit mTORC1 and induce autophagy.
Oxidative stress can also promote autophagy through NF-κB-mediated upregulation of p62/SQSTM1.
Immune signal
Immune signaling can regulate the autophagy pathway in the TME. Damage-associated molecular patterns ( DAMPs ) and cytokines are the main mediators that regulate the autophagic response.
Extracellular DAMP signals are sensed by extracellular or intracellular pattern recognition receptors, such as Toll-like receptors ( TLRs ), and autophagy induction occurs when various TLRs recognize DAMPs and activate downstream signals.
Furthermore, in Drosophila, cytokines such as TNF and IL-6-like signaling can activate autophagy, thereby promoting early tumor growth and invasion.
TGF-β increases the transcriptional levels of BECN1, ATG5, and ATG7 and activates autophagy through SMAD-dependent and SMAD-independent pathways, which can delay the apoptosis of human hepatocellular carcinoma and breast cancer cells in vitro.
Autophagy-mediated immune evasion
Evasion of antitumor immune responses is an important survival strategy for various tumors.
Recent evidence suggests that autophagy plays an important role in tumor immune evasion.
It has been found that the downregulation of MHC class I molecules in pancreatic ductal adenocarcinoma ( PDAC ) is mediated through selective autophagic degradation, and inhibition of autophagy unleashes a strong anti-tumor immune response.
On the other hand, MDSCs play an immunosuppressive role in the TME, and studies have demonstrated that autophagy in MDSCs is a key mechanism for suppressing the antitumor immune activity of melanoma.
Autophagy in MDSC immune cells is central to the degradation of MHC class II molecules, preventing the priming and activation of antitumor T cells.
Autophagy and drug resistance
Autophagy, as a mechanism by which ( cancer ) cells respond to threatening stressors, is considered to be an important mechanism of therapy resistance in cancer therapy.
There is already evidence that tumor cell resistance to cisplatin is mediated, at least in part, by increased autophagy in ovarian cancer cell lines.
Similar evidence suggests that cisplatin, doxorubicin, and methotrexate overcome chemoresistance by inhibiting autophagy in osteosarcoma.
Interestingly, for example, the interaction between cisplatin and autophagy is a continuum, even in vitro and in vivo in cells that are not inherently resistant to certain chemotherapeutic drugs, with the addition of autophagy inhibitors such as chloroquine ( CQ ) can also increase healing.
This has been demonstrated in mouse models of adrenocortical carcinoma, colon cancer cell lines and in 5-fluorouracil- and temozolomide-induced cytotoxicity of glioma cells.
Apart from this, similar results have been performed for antibody-based therapy, for example in trastuzumab-resistant breast cancer cells, autophagy inhibition using CQ led to almost complete tumor elimination.
Similarly, inhibition of autophagy using CQ was also effective against bevacizumab-induced autophagy in colorectal cancer cells and reduced tumor growth in an in vivo mouse tumor model.
Research status of autophagy in tumor therapy
Autophagy inhibitors are divided into early inhibitors targeting ULK1/ULK2 or VPS34, such as SBI-0206965, 3MA, and wortmannin, and late inhibitors targeting lysosomes, such as CQ, hydroxychloroquine ( HCQ), bafemycin A1 and monensin, CQ and HCQ inhibit autophagosome degradation by interfering with lysosomal acidification.
However, in clinical trials, HCQ monotherapy failed to control tumor growth in patients with advanced pancreatic cancer.
At present, autophagy inhibition is often combined with other cancer treatments to improve the therapeutic effect.
Chemotherapy
High autophagic flux in cancer is associated with decreased response to chemotherapy and is associated with poor survival in cancer patients.
Preclinical studies have shown that inhibition of autophagy can overcome chemotherapy resistance in NSCLC, bladder cancer, thyroid cancer, and pancreatic cancer.
In addition, results from several studies suggest that autophagy inhibition may synergize with inhibition of MEK–ERK signaling.
An earlier phase II study in 2014 used HCQ monotherapy in patients with metastatic pancreatic cancer who had previously been treated with other methods, with a primary endpoint of two-month progression-free survival.
As a result, autophagy levels were reduced to varying degrees in different patients, but the primary endpoint was not significantly improved.
Another study combining HCQ, gemcitabine, and nab-paclitaxel in patients with advanced or metastatic pancreatic cancer also failed to demonstrate a 12-month increase in overall survival. Importantly, however, patients taking HCQ showed a significantly better response rate ( 38.2% vs 21.1% ).
Radiotherapy
Autophagy plays a key role in protecting tumor cells from radiation-induced cell death.
In breast cancer cells, radiation induces the expression of autophagy-related genes, accompanied by the accumulation of autophagosomes.
Short-term inhibition of autophagy concomitant with radiotherapy enhances the cytotoxicity of radiotherapy against drug-resistant cancer cells. Likewise, hypoxia enhanced the radioresistance of A549 lung cancer cells by inducing autophagy.
In glioblastoma, radiation therapy induces autophagy by increasing the expression of mammalian STE20-like protein kinase 4 ( MST4 ), which stimulates autophagy through ATG4B phosphorylation.
The small molecule inhibitor NSC185058 ( targeting ATG4B ) combined with radiotherapy impairs intracranial xenograft growth of glioblastoma and prolongs survival in treated mice.
Therefore, targeting tumor autophagy may enhance the efficacy of radiation therapy.
In fact, autophagy inhibitors have been utilized in combination with radiation therapy in clinical trials in cancer patients.
Immunotherapy
Harnessing the immune system is an important way to fight cancer. Inhibition of autophagy may impair systemic immunity, as autophagy is involved in immune system development and the survival and function of effector T cells.
However, systemic inhibition of autophagy by CQ over a short period of time did not impair T cell function in preclinical models of melanoma and breast cancer.
The data suggest that the immune system may be tolerant to some degree of autophagy inhibition. However, given that autophagy can regulate tumor immune responses, targeting autophagy can improve the efficacy of immunotherapy and overcome immunotherapy resistance.
For example, inhibition of VPS34 kinase activity using the inhibitors SB02024 or SAR405 resulted in increased levels of CCL5, CXCL10, and IFN-γ in the TME, leading to increased levels of NK and T cell tumor infiltration in melanoma and colorectal cancer models. In these models, VPS34 inhibition also reversed resistance to anti-PD1 or anti-PD-L1 therapy.
Furthermore, CQ treatment, which blocks autophagy-mediated degradation of MHC class I, synergized with dual ICB treatment ( anti-PD1 and anti-CTLA4 antibodies ), produced enhanced antitumor immune responses in a mouse model of pancreatic cancer.
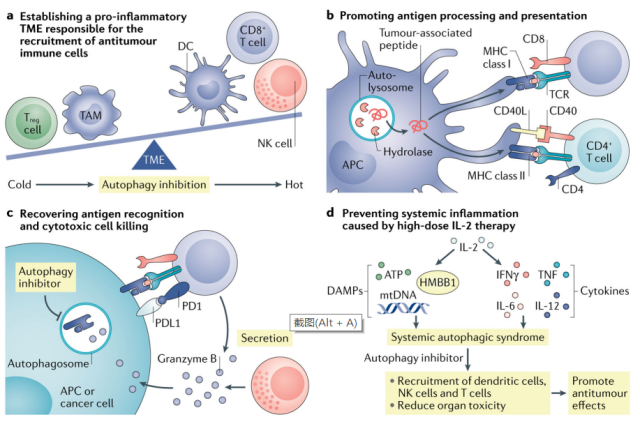
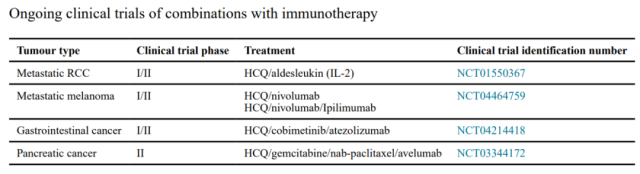
Therefore, targeting autophagy may enhance immunotherapy. Clinical trials of HCQ combined with immunotherapy in patients with different types of cancer are currently underway.
In addition, CAR-T cell therapy has achieved clinical success in the treatment of blood cancers, but still has limited efficacy in the treatment of solid cancers.
Autophagy modulation may provide some benefits to cancer patients treated with CAR-T cells.
It is well known that the TME is a barrier to the infiltration and function of CAR-T cells in solid tumors.
Given that autophagy inhibition can remodel the TME and promote the production of TH1-type chemokines, autophagy inhibition can promote the translocation of CAR-T cells to tumors. -Enhanced autophagy in T cells may support T cell fitness and survival in the TME.
In addition, tumor autophagy inhibition may lead to increased antigen expression, thereby enhancing CAR-T cell-mediated tumor killing. Finally, autophagy inhibition may ameliorate cytokine release syndrome and bring clinical benefit to patients.
Overall, the potential to inhibit autophagy to enhance the efficacy of immunotherapy is a promising area of ongoing exploration.
Summary
Autophagy is an important mechanism of research in many fields such as tumor biology and immunology.
Given that different factors in different cells in the TME can induce autophagy, its induction and activation can promote or inhibit tumor progression.
Autophagy in T cell subsets may play an active role in antitumor immune responses, while functional autophagy in tumor cells may support tumor antigen presentation and recognition, in which case inhibition of autophagy may be detrimental to antitumor immunity. immunity.
On the other hand, the autophagy pathway may be related to tumor cell survival, tumor antigen degradation, reduction of TH1-type chemokines, and enhancement of Treg cells and MDSCs. Therefore, systemically targeting autophagy for cancer therapeutics is challenging.
Therefore, in order to target the autophagy pathway in tumor immunotherapy, it is crucial to explore the biological activity of autophagy in major immune cell subsets.
Recent studies have begun to evaluate the autophagy pathway in T cells, macrophages, and dendritic cells. Future work may extend to B cells, NK cells, and NKT cells.
Furthermore, as cancer progresses, autophagy pathways dynamically change in response to different stimuli in the TME, and it is imperative to understand how autophagy simultaneously participates in the function and survival of immune cells, tumor cells, and stromal cells in the TME in order to Can ultimately enable cancer patients to obtain clinical benefits.
references:
1. Autophagy in tumor immunity and therapy. NatRev Cancer. 2021 May; 21(5): 281–297.
2. Autophagy in Cancer Therapy-Molecular Mechanisms and Current Clinical Advances. Cancers (Basel). 2021 Nov8;13(21):5575.
Autophagy at the forefront of tumor immunotherapy
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



