New strategy for tumor immunotherapy: Lactate metabolism in Treg cells
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
New strategy for tumor immunotherapy: Lactate metabolism in Treg cells
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
New strategy for tumor immunotherapy: Lactate metabolism in Treg cells.
“Metabolic imbalance” has long been regarded as one of the hallmarks of tumors [1] .
Specifically, cancer cells can utilize glucose to promote aerobic glycolysis for survival (called the “Warburg effect”) [1] .
In such a low-glucose, high-lactate microenvironment, it is difficult for T cells to function normally [2] .
For Treg cells, specific metabolic reprogramming occurs in the tumor microenvironment [3-4] .
But there are still many key scientific questions that have not been elucidated: How do metabolites in the tumor microenvironment directly act on Treg cells? Do Treg cells have specific metabolic checkpoints in response to specific metabolic microenvironments? The above problems still need to be systematically explored.
The research group of Professor Hiroyoshi Nishikawa from the National Cancer Research Center of Japan published a research paper titled Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments on Cancer Cell , and found that lactic acid can promote the growth of highly glycolytic tumor microenvironments.
Treg cells were induced to express PD-1 in MCT, and MCT1 was found to be an important metabolic checkpoint in this process, providing new clues for in-depth understanding of the metabolic and immune “balance” of the tumor microenvironment.

In order to explore the relationship between the metabolic characteristics of tumors and effector Treg cells (eTreg cells) , the researchers conducted in-depth studies on gastric cancer and non-small cell lung cancer samples using RNA-seq and flow cytometry, and found that eTreg highly infiltrated Tumor tissue exhibits the characteristics of hyperglycolytic metabolism and MYC activation.
The authors further found that tumor tissues with high glycolysis/high expression of MYC exhibited high infiltration of PD-1+eTreg cells, while PD-1+CD8 + T cells conversely had low infiltration, suggesting that tumor metabolites may function on eTreg cells play an important role.
Given that lactate is the end product of tumor glycolysis, the authors found that the lactate transporter MCT1 (Slc16a1) was specifically enriched in PD-1+ eTreg, but not in CD8 + T cells.
ChIP-seq showed that Treg cell-specific transcription factor FOXP3 can directly bind to the DNA of MCT1.
These results suggest that lactate may play an important role in the function and phenotype of Treg cells.
Therefore, we explored the association between lactate metabolism and PD-1 expression in eTreg cells. Lactate gradient stimulation showed that PD-1 expression in eTreg cells significantly increased with increasing lactate concentration, whereas CD8 + T cells showed an opposite trend.
Mechanistically, the authors believe that when Treg cells take up lactate from the microenvironment through MCT1, lactate may be metabolized in Treg cells to phosphoenol pyruvate (PEP) , which is exactly T cells. immune metabolic checkpoints [5] .
Therefore, we explored the correlation between lactate concentration and phosphoenolpyruvate and found that lactate increased eTreg phosphoenolpyruvate, but not in CD8 + T cells.
The authors further used MCT1 inhibitor to explore its relationship with T cell phenotype and found that PD-1 expression in eTreg cells was significantly reduced in a concentration-dependent manner under high lactate conditions, and MCT1 inhibitor could reduce eTreg proliferation and enhance eTreg apoptosis.
These results suggest that eTreg cells are more suppressive at high lactate concentrations. PD-1 expression in Treg cells was significantly reduced in MCT1-deficient mice (Slc16a1+/- mice) .
These results suggest that a high lactate environment enables eTreg cells to utilize MCT1 to uptake lactate to upregulate PD-1, thereby affecting eTreg cell function and phenotype.
Finally, the authors systematically explored the scientific question of “interaction between tumors and Treg cells”.
First, the authors found that overexpression of MYC produced higher levels of lactate and increased PD-1 expression in Treg cells in vitro and in vivo; whereas knockdown of LDHA, a key enzyme in glucose metabolism, in MYC-overexpressing cells significantly reduced lactate production and reversed Treg cells Suppression phenotype, and these results are more consistent in MC-38 intestinal cancer model, B16 melanoma model and liver metastasis model. Further in vivo inhibition of MCT1 can reduce the proportion of Treg cells in the microenvironment, inhibit the expression of PD-1 in Treg cells, and enhance the efficacy of anti-PD-1 mAbs. Importantly, MYC and LDHA, a key enzyme in glucose metabolism, can predict the efficacy of immunotherapy in patients with gastric cancer, non-small cell lung cancer, and melanoma in independent clinical cohorts, and are closely related to the clinical outcomes of patients.
Taken together, this work found that hyperglycolytic tumors can release excess lactate, thereby promoting lactate uptake by Treg cells through MCT1 to enhance PD-1 expression and remodel Treg cell function and phenotype, which may lead to One of the reasons for the resistance to αPD-1 immunotherapy.
This study provides new clues for designing novel immunotherapeutic strategies: targeting Treg cell-specific immune metabolic checkpoints may serve as potential therapeutic strategies.
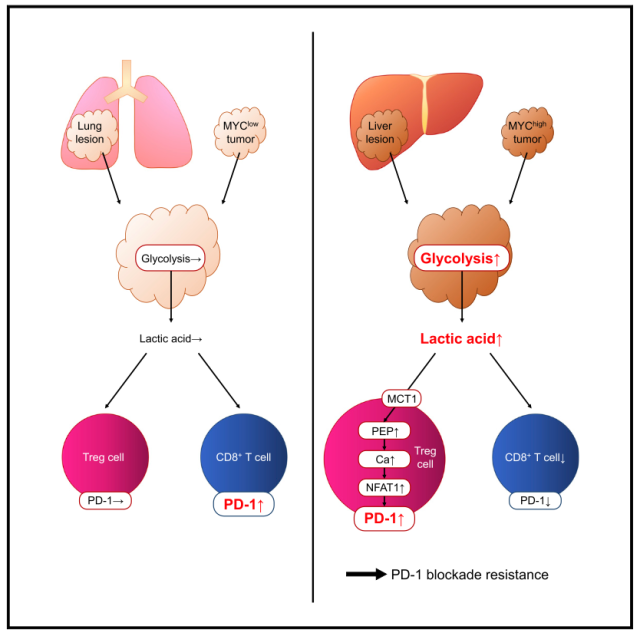
The main findings of this study are that hyperglycolytic tumors can release excess lactate, thereby promoting lactate uptake by Treg cells through MCT1 to enhance PD-1 expression and remodel Treg cell function and phenotype
Original link:
https://doi.org/10.1016/j.ccell.2022.01.001
References:
Original Paper link: https://doi.org/10.1016/j.ccell.2022.01.001
[1] Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12(1):31-46. doi:10.1158/2159-8290.CD-21-1059
[2] Ho PC, Kaech SM. Reenergizing T cell anti-tumor immunity by harnessing immunometabolic checkpoints and machineries. Curr Opin Immunol. 2017;46:38-44. doi:10.1016/j.coi.2017.04.003
[3] Angelin A, Gil-de-Gómez L, Dahiya S, et al. Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab. 2017;25(6):1282-1293. e7.doi:10.1016/j.cmet.2016.12.018
[4] Zappasodi R, Serganova I, Cohen IJ, et al. CTLA-4 blockade drives loss of Treg stability in glycolysis-low tumors. Nature. 2021;591(7851):652-658. doi:10.1038/s41586-021 -03326-4
[5] Ho PC, Bihuniak JD, Macintyre AN, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162(6):1217-1228. doi:10.1016/j.cell.2015.08 .012
New strategy for tumor immunotherapy: Lactate metabolism in Treg cells
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



