Immunotherapy: How to use Gamma Delta T cells in tumor treatment?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Immunotherapy: How to use Gamma Delta (γδ) T cells in tumor treatment?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Immunotherapy: How to use Gamma Delta (γδ) T cells in tumor treatment?
Preface
At present, immunotherapy has become the fourth pillar of anti-tumor therapy in addition to surgery, chemotherapy and radiotherapy. Among them, T cell-based immunotherapy is an effective cancer treatment strategy.
According to the expression of αβ and γδ T cell receptors (TCR), T cells can be divided into two main subgroups. αβT cells recognize non-self peptide antigens, such as antigens expressed by cancer cells. αβT cells are effector cells of adaptive immunity.
These cells exert cytotoxicity in a manner restricted by the major histocompatibility complex (MHC). However, due to the loss of MHC molecules, tumor cells usually resist the attack of αβT cells.
In contrast, γδ T cells are effector cells in the innate immune system. These cells function in an unrestricted MHC manner, making them an ideal medium for cancer immunotherapy. Recent studies have shown that γδT cells have a strong cytotoxic effect on various types of cancer cells.
However, γδ T cells account for only a small part of circulating lymphocytes, and the clinical benefit is not satisfactory. At present, some improved methods, such as bispecific antibodies and CAR-T, may break through the limitations of γδ T cells and improve the anti-tumor effect.
γδ T cell activation
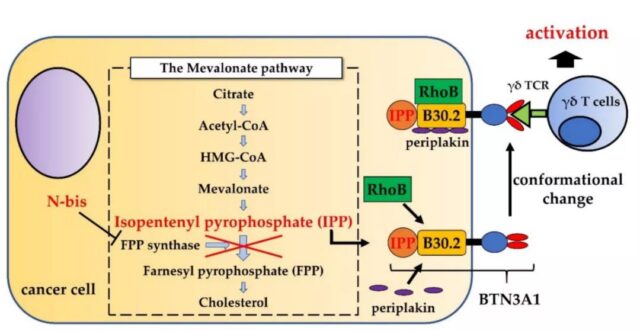
Human peripheral blood γδ T cells mainly express Vδ2 chains and Vγ9 chains, and are activated when they recognize phosphorylated antigens (PAG), such as (E)-4-hydroxy-3-methyl-2-enyl pyrophosphate (HMBPP). BTN3A1 is a subtype of the BTN3A (CD277) family and plays an indispensable role in PAG activation of γδ T cells. BTN3A1 is widely expressed on the cell surface and consists of two immunoglobulin-like extracellular domains and one intracellular B30.2 domain.
Generally speaking, under physiological conditions, the concentration of PAG is not sufficient to stimulate γδ T cells. However, due to metabolic reprogramming, tumor cells exhibit up-regulation of PAG production, which increases the activity of the mevalonate pathway. In addition, the concentration of PAG can be increased pharmacologically. Nitrogen-containing bisphosphonates used to treat hypercalcemia or cancer bone metastasis, such as pamidronate (Pam) and zoledronate (ZOL), can inhibit farnesyl diphosphate (FPP) synthase , This is the rate-limiting enzyme in the mevalonate pathway. Therefore, the concentration of IPP (upstream metabolite of FPP) increases, thereby activating γδ T cells.
Other interactions between γδT cells and cancer cells
γδ T cells not only recognize PAG through γδTCR, but also recognize stress-related antigens through NKG2D receptors. For natural killer cells, this recognition method is not restricted by MHC. MICA is a functional ligand that stimulates NKG2D receptors. In addition to MICA, the interaction between MICB and ULBP1-4 in human NKG2D ligands and NKG2D receptors can recognize cancer cells and γδT cell-mediated cytotoxicity Very important.
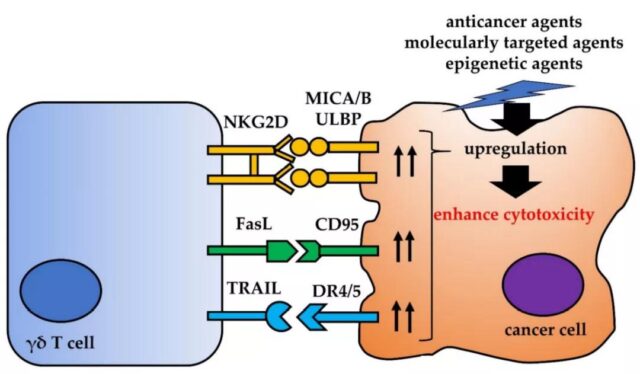
In activated γδ T cells, the expression of Fas ligand (FasL) and TNF-related apoptosis-inducing ligand (TRAIL) was up-regulated. FasL interacts with CD95 (also known as Fas or APO-1). FasL activates the caspase cascade after binding to CD95, thereby initiating cancer cell apoptosis.
TRAIL interacts with five receptors (TRAILRs): DR4, DR5, DcR1, DcR2 and osteoprotegerin. The death receptors DR4 and DR5 contain an intracellular domain called the death domain. When these receptors bind to TRAIL, this region enables these receptors to initiate cytotoxicity signals. Therefore, the up-regulation of CD95 or death receptor DR4 or DR5 in tumor cells may enhance the cytotoxicity mediated by γδ T cells.
Tumor microenvironment and γδT cells
Some studies have proved the plasticity of γδ T cells. After activation by PAGs, γδ T cells promote Th1 immune response by secreting TNF-α and IFN-γ. However, γδ T cells can also be polarized into cells with similar properties to Th2 cells, Th17 cells, or regulatory T cells (Treg).
Tumors include not only cancer cells, but also extracellular matrix (ECM), stromal cells (such as fibroblasts and mesenchymal stromal cells), vascular network and immune cells, such as T cells and B cells, NK cells, and various immune cells. Suppressor cells, such as tumor-associated macrophages (TAM), myeloid-derived suppressor cells (MDSCs), etc., constitute the tumor microenvironment (TME).
TME contains various cytokines, chemokines, growth factors, inflammatory mediators and matrix remodeling enzymes to promote crosstalk between the cells that make up TME; this environment can promote the polarization of γδ T cells into Th17 or Treg-like cells, Produce IL-17 and TGF-β, which is conducive to the proliferation of cancer cells. Γδ T cells that produce IL-17 induce angiogenesis and support cancer progression, and TGF-β can negatively regulate γδ T cells.
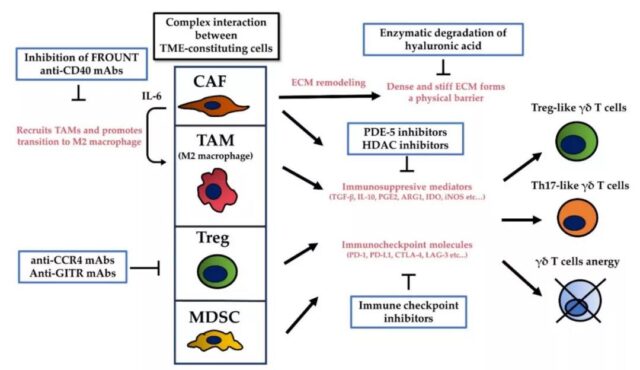
Targeting TME therapy can enhance the anti-tumor effect by activating and improving the cytotoxicity of γδT cells. Among these TME targeted therapies, therapeutic antibodies against suppressive immune checkpoint molecules are an effective means to overcome the immunosuppressive effects of TME. The combination of adoptive γδ T cells and immune checkpoint inhibitors is a promising strategy to improve their cytotoxicity, because PAG-stimulated γδ T cells express PD-1, and blocking PD-1 may enhance the anti-tumor effect of γδ T cells.
New form of γδ T cell therapy
In recent years, in order to improve the anti-tumor effect of γδT cell immunotherapy, several strategies have been proposed. The use of bispecific antibodies significantly improves cytotoxicity. Preclinical studies have found that EpCAM/CD3 bispecific antibodies enhance γδT cell-mediated lysis of hepatoblastoma and childhood hepatocellular carcinoma. In preclinical models, in vitro expansion of γδ T cells with HER2/Vγ9 bispecific antibody can significantly reduce the growth of pancreatic cancer and colon cancer. In addition, a tribody (HER22xCD16) is reported, which contains two HER2-specific single-chain fragments fused with a single-chain fragment of CD16 expressed on γδ T cells and NK cells, which enhances the mediation of γδ T cells and NK cells. The HER2 expresses the lysis of tumor cells.
Chimeric antigen receptor-transduced γδT cells (CAR-γδT) is another new strategy to overcome the limitations of current treatments. Preclinical studies found that compared with negative CAR-γδ T cells in vitro, CAR-γδ T cells with CD19 specificity enhanced the killing of CD19+ tumor cells and reduced CD19+ leukemia xenografts in a mouse model.
CAR-T cell immunotherapy has the problem of targeting effect. Fisher et al. designed GD2-specific CAR-γδ T cells to limit the toxic effects on normal cells. GD2 is overexpressed on the surface of neuroblastoma cells and several other cancer cells. In this study, γδ T cells recognize tumor PAG antigen, and then anti-GD2-CAR recognizes GD2 and activates downstream signal domains to exert anti-tumor effects. Therefore, GD2-expressing neuroblastoma cells that bind to γδTCR are effectively lysed, while GD2-expressing cells that bind to γδTCR are not affected.
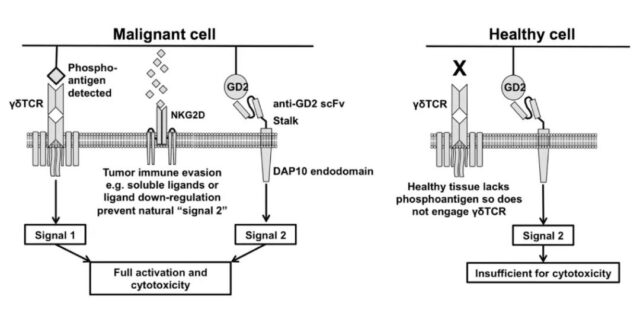
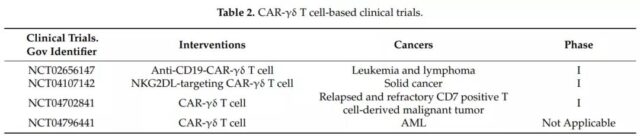
Currently, some clinical studies are underway. CAR-γδ T cells are expected to become a new type of γδ T cell immunotherapy. picture
Summary
Immunotherapy based on γδ T cells is very attractive. These cells show strong cytotoxic effects on various types of cancer in vitro and in mouse models. However, clinical trials have shown limited clinical benefits.
New methods, including γδ T cells and immune checkpoint inhibitor combined immunotherapy, bispecific antibodies and CAR-γδ T cells are all new strategies that are expected to overcome the limitations of current treatments.
Further research on the immunosuppressive effect of TME on γδ T cells, as well as the clinical research of anti-cancer drug combination therapy, molecular targeted drugs, epigenetic drugs, bispecific antibodies and CAR-γδ T cells, will be the future clinical research of γδ T cell immunotherapy The application lays the foundation.
Immunotherapy: How to use Gamma Delta T cells in tumor treatment
References:
1.Strategies to Improve the Antitumor Effect of γδT Cell Immunotherapy for Clinical Application. Int J Mol Sci. 2021Aug; 22(16): 8910.
Immunotherapy: How to use Gamma Delta T cells in tumor treatment
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



