How to use NK Cells for Cancer Immunotherapy?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
How to use NK Cells for Cancer Immunotherapy?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
How to use NK Cells for Cancer Immunotherapy?
Natural Killer (NK) cells are a type of innate lymphocytes that have the ability to find and rapidly kill infected, cancerous, foreign or stressed cells.
NK cells have received extensive attention in recent years.
They can not only directly kill tumor cells, but also rapidly express a variety of cytokines and chemokines, recruit other immune cells and promote adaptive immune responses of T cells and B cells.
Moreover, NK cells can be activated by IgG antibodies, resulting in antibody-dependent cell-mediated cytotoxicity (ADCC) .
In addition, it rarely stimulates an autoimmune response, but instead promotes immune balance and fights autoimmune diseases.
The various beneficial characteristics of NK cells make them ideal targets for immunotherapy.
Recently, the journal Nature Reviews Drug Discovery published a review paper titled: Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders , which reviewed various strategies for using NK cells to develop anti-cancer immunotherapy.

The biology of human NK cells
NK cells belong to type 1 innate lymphocytes, and one of its main features is the production of type 1 cytokines, including IFNγ and tumor necrosis factor (TNF) .
The activity of NK cells is regulated by a variety of activating and inhibitory receptors expressed on the cell surface . Most healthy cells express the major histocompatibility complex type I (MHC-I) , which stimulate potent inhibitory signals to suppress NK cell activity.
While tumor cells often evade recognition by CD8-positive cytotoxic T cells by reducing MHC-I expression, loss of MHC-I ligands and/or increased expression of activating receptor ligands can alter signaling within NK cells balance, causing NK cells to activate and attack target cells with which they come into contact.
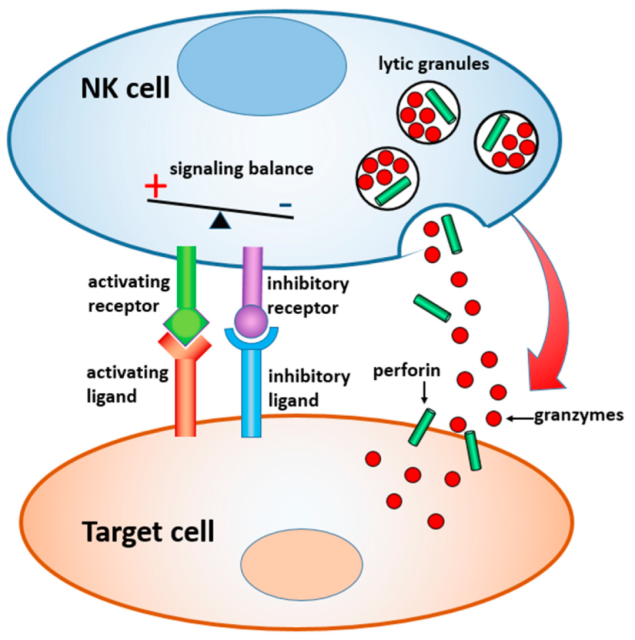
The mechanism of action of NK cells
In peripheral blood, NK cells can easily access hematological tumor cells, and solid tumors are relatively more difficult to reach and infiltrate, and the tumor microenvironment with immunosuppressive ability can drive NK cells into a state of exhaustion.
Currently, researchers are using a variety of means to enhance the function of NK cells.
Strategies to enhance NK cell function
Cytokines that stimulate NK cells
Antagonists of various activating or inhibitory cytokines are currently being evaluated in clinical trials. For example, IL-2 can directly stimulate the proliferation and activation of T cells and NK cells.
IL-2 is frequently used to activate NK cells in vitro, however its use in vivo is limited by toxic side effects and activation of immunosuppressive regulatory T cells (Treg) .
Several companies are developing IL-2 analogs that selectively activate T cells and NK cells.
For example, SAR444245 developed by Sanofi, nemvaleukin alfa (ALKS 4230) developed by Alkermes , and ANV419, a selective IL-2R agonist developed by Anaveon, etc.
IL-15 is one of the cytokines that is expected to replace the function of IL-2, it can stimulate NK and CD8 positive T cells, but not Treg cells.
Currently, several IL-15 recombinant proteins are being tested in clinical trials.
Among them, ImmunityBio’s IL-15 super agonist Anktiva (N-803)has achieved positive results in clinical trials for the treatment of bladder cancer, indolent non-Hodgkin’s lymphoma and other diseases.
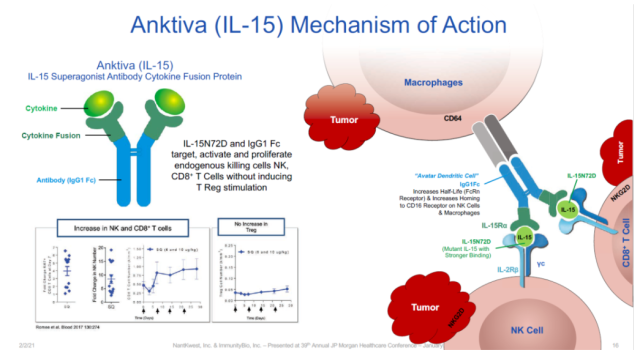
The mechanism of action of Anktiva (Image source: ImmunityBio’s official website)
Other cytokines that activate NK cells include IL-12, IL-18, IL-21, and TGF[beta] inhibitors.
Antibodies that elicit ADCC responses
A typical effector function of NK cells is the killing of cells targeted by IgG1 or IgG3 antibodies through CD16 receptor-mediated ADCC.
The different morphology of CD16 on the surface of NK cells affects the affinity of CD16 with the Fc-terminal of the antibody.
Specifically, when the amino acid at position 158 of the CD16 protein is phenylalanine (158F), the affinity for Fc is low, and when the amino acid at this position is valine(158V), the affinity for Fc is high.
Many of the new generation of monoclonal antibody therapies have modified the Fc-terminal to improve their binding to CD16 and promote ADCC production.
At the same time, many antibodies were synthesized using a mammalian culture system that prevented fucosylation of the Fc terminal of the antibody, which enhanced their binding to CD16.
Bispecific or specific antibodies
Bispecific or trispecific antibodies can simultaneously bind to NK cell-activated receptors and tumor antigens, promoting more potent and durable NK-mediated cytotoxicity.
Unlike common mAbs, these fusion proteins can be designed to bind to a variety of tumor antigens and NK receptors, and can utilize antibody variable region fragments (Fv)to bind to CD16, ensuring that they can bind to 158V C16 and 158F C16 binds efficiently. Similar protein designs have been used in bispecific T cell engagers(BiTEs).
Currently, one of the most rapidly advancing CD16 bispecific antibodies is AFM13 developed by Affimed. This is a bispecific antibody targeting CD30.
In a phase 1 clinical trial in patients with Hodgkin and non-Hodgkin lymphoma, it was combined with cord blood-derived NK cells to achieve a 100% objective response rate in 12 patients treated at the recommended dose .
Trispecific NK cell adaptors can not only bind to tumor antigens and CD16 receptors, but also contain IL-15, thereby enhancing the activity of NK cells by binding to IL-15 receptors on the surface of NK cells .
Trispecific antibodies are emerging as an exciting frontier in NK cell immunotherapy due to their potential to simultaneously bind to different NK cell receptors.
However, which activating receptor combinations can maximize the anticancer activity of NK cells still needs more research.
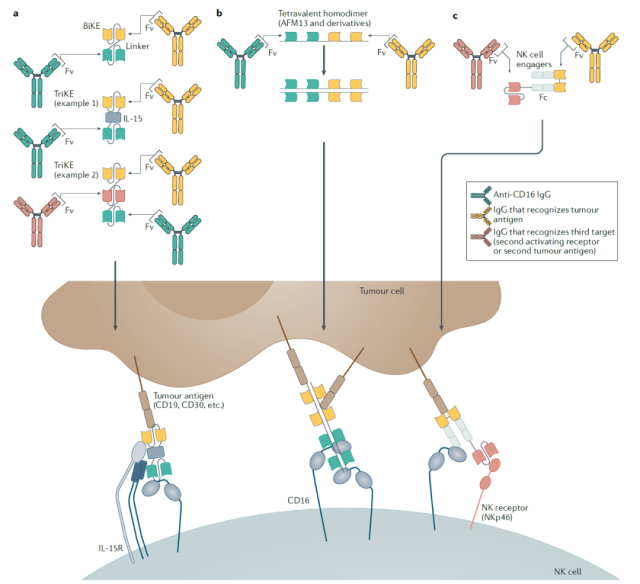 Bispecific and trispecific antibodies that enhance NK cell function
Bispecific and trispecific antibodies that enhance NK cell function
Adoptive NK cell therapy
Most adoptive NK cell therapies harvest NK cells from various sources, enhance their function in vitro, and then infuse them into cancer patients.
Both autologous and allogeneic NK cells can be used as a source of adoptive NK cell therapy.
There are various sources of allogeneic NK cells, including peripheral blood NK cells, NK cells from umbilical cord blood or placenta, and NK cells differentiated from induced pluripotent stem cells (iPSCs) .
Some studies have shown that NK cells derived from umbilical cord blood are easier to culture and activate, whereas NK cells derived from adult peripheral blood have a more natural cytolytic capacity.
NK cell lines (such as NK92) and iPSC cell lines may provide a source of NK cells that can be regenerated repeatedly.
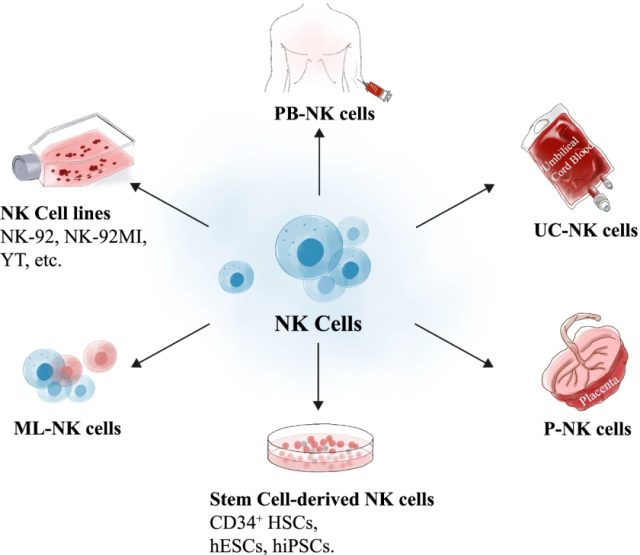 Different sources of NK cells
Different sources of NK cells
A straightforward approach to enhancing NK cell function is in vitro stimulation with cytokines, historically the most commonly used cytokine is IL-2.
In recent years, other stimulatory factors such as IL-12, IL-15, IL-18, and IL-21 have also been used to stimulate NK cells in vitro, and can generate long-term survival and memory NK cells.
The CD16/CD30-targeting bispecific antibody AFM13 developed by Affimed can also be used to pre-bind with NK cells in vitro to promote NK cell responses to CD30-positive lymphomas.
Expression of chimeric antigen receptors (CARs) targeting tumor antigens on the surface of NK cells can confer the ability of NK cells to target specific tumors.
CAR-NK cells may offer several advantages over CAR-T cells, including a reduced risk of cytokine release syndrome.
And even if CAR-NK cells lose CAR, they can still recognize and kill tumor cells through intrinsically expressed activating receptors.
In addition to the introduction of CAR, researchers are exploring other strategies for genetic engineering to enhance NK cell function.
These include promoting tumor infiltration by expressing chemokine receptors.
ADCC-based therapy can express the high-affinity 138V CD16 receptor on NK cells and combine with antibodies that stimulate ADCC to constitute combination therapy.
Finally, side-regulators can be knocked out from NK cells using the CRISPR-Cas9 gene editing system. These approaches offer multiple possibilities for building better NK cell therapies.
Summary and Outlook
The review authors note that while the development of NK cells as an anticancer immunotherapy has gained momentum in recent years, the field is still in its infancy and the future is bright for developing new approaches to further enhance the NK cell immunotherapy platform.
Important goals to be addressed include improving the targeting specificity of NK cells to solid tumors and how to enhance their activation, cytolytic capacity, and survival when NK cells reach an immunosuppressive tumor microenvironment.
CARs, advanced antibody engineering, and multiple receptor binding technologies offer a wide range of opportunities to optimize NK cell activation.
Combination therapies focused on NK cells will provide the next wave of clinical advances.
Reference:
https://www.nature.com/articles/s41573-022-00413-7
How to use NK Cells for Cancer Immunotherapy?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



