Positive Phase 1 Results for PRGN-2012 Vaccine in HPV-Related Cancer Treatment
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Positive Phase 1 Results for PRGN-2012 Vaccine in HPV-Related Cancer Treatment
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Positive Phase 1 Results for PRGN-2012 Vaccine in HPV-Related Cancer Treatment.
Recurrent Respiratory Papillomatosis (RRP), a rare and debilitating tumor disease, caused by chronic infection with HPV types 6 or 11, is characterized by the repeated growth of papillomas in the respiratory and digestive tracts.
Unlike other HPV-related cancers, RRP lacks driving mutations, indicating that HPV-related cellular dysfunction is the primary cause of RRP.
Currently lacking approved treatment drugs, immune checkpoint blockade therapy (ICB) has not demonstrated sustained enhancement of HPV-specific T-cell activity and lasting complete remission. Patients are forced to undergo dozens or even hundreds of surgeries throughout their lives to control tumor growth.
This underscores the necessity of developing new treatment methods that safely and effectively induce a robust HPV-specific T-cell response to control the disease’s progression.
Recently, researchers from the National Institutes of Health in the United States published a study in the journal Science Translational Medicine titled “The tumor microenvironment state associates with response to HPV therapeutic vaccination in patients with respiratory papillomatosis.”
This first-in-human clinical trial aimed to explore the safety, clinical activity, and immune-related outcomes of the therapeutic vaccine PRGN-2012. Developed by Precigen, PRGN-2012 is an adenovirus vector immunotherapy expressing a composite protein antigen designed to induce a reactive T-cell response against HPV-6 and HPV-11.
The Phase 1 clinical trial results indicate that PRGN-2012 is well-tolerated, with a 50% complete remission rate observed in the high-dose group. Responders showed a greater expansion of peripheral HPV-specific T-cells compared to non-responders.
This study suggests that therapeutic vaccines can enhance the immune system’s defense against Recurrent Respiratory Papillomatosis (RRP), bringing new hope to patients suffering from this disease with limited treatment options.
It is well-known that HPV is the primary culprit behind cervical cancer, but chronic infections caused by HPV types 6 or 11 can also lead to a rare cancer—RRP. Since there is currently no treatment for this disease that lacks driving mutations, the only available treatment method is repeated surgeries to control tumor growth, which does not address the underlying biological issues causing its occurrence.
Therapeutic vaccines can induce antigen-driven T-cell responses and clinical activity in virus-driven diseases. Using a non-human adenovirus vaccine platform with a large gene payload capacity, researchers can develop T-cell responses targeting multiple antigenic targets.
In this clinical trial, the research team tested a therapeutic vaccine using an adenovirus vector derived from gorillas. The inactivated adenovirus delivered the vaccine payload to the cells of 15 participants. These participants, suffering from recurrent respiratory papillomatosis (RRP) for many years with ineffective previous treatments, were divided into two groups: a low-dose group of 3 individuals receiving an injection of 1×10^11 virus particles and a high-dose group of 12 individuals receiving an injection of 5×10^11 virus particles.
These participants underwent 3 to 10 surgical interventions in the previous 12 months, with a median of 6 surgeries. Partial relief (PR) was defined as a 50% or more reduction in the need for surgical interventions in the 12 months following vaccine administration, and complete relief (CR) was defined as no longer requiring surgical interventions.
The results showed that participants tolerated the vaccine well, experiencing only mild side effects. In the low-dose treatment group, one patient achieved partial relief (33%), while in the high-dose group of 12 patients, seven achieved partial or complete relief (58%), with six achieving complete relief (50%). Among the 15 participants, the overall relief rate was 53% (8 with relief, 7 with no response).
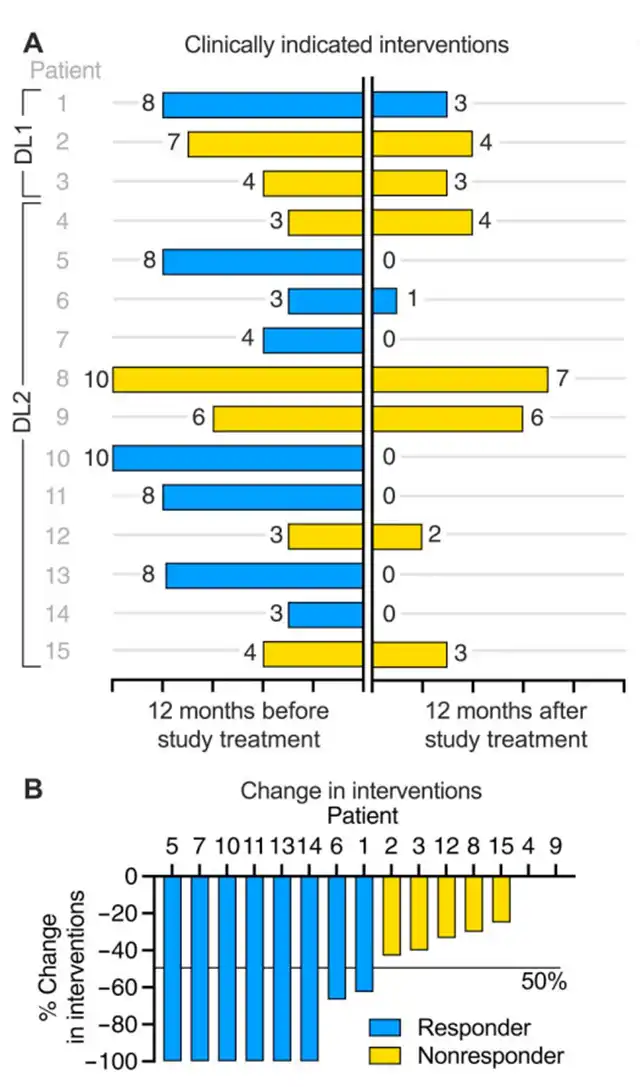
Further analysis indicated that this beneficial response is associated with the tumor microenvironment, with the vaccine exerting its effects by expanding peripheral HPV-specific T-cells. These results suggest the need for further clinical development of PRGN-2012 in RRP patients, with a Phase 2 clinical trial currently underway.
Link to the paper: https://www.science.org/doi/10.1126/scitranslmed.adj0740
Positive Phase 1 Results for PRGN-2012 Vaccine in HPV-Related Cancer Treatment
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



