Safety Study of GLP-1 Class Drugs During Pregnancy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Safety Study of GLP-1 Class Drugs During Pregnancy
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Safety Study of GLP-1 Class Drugs During Pregnancy
Semaglutide, initially developed by Novo Nordisk as a glucose-lowering drug for type 2 diabetes, is a glucagon-like peptide-1 (GLP-1) receptor agonist. It mimics the action of GLP-1, reducing hunger, decreasing food intake, and lowering calorie consumption, making it highly effective for weight loss. In June 2021, the FDA approved semaglutide for marketing as a weight-loss drug under the name Wegovy, quickly gaining popularity worldwide.
Babies born to women with pre-existing diabetes before pregnancy are at a higher risk of congenital abnormalities. In recent years, the use of new antidiabetic drugs like GLP-1 receptor agonists has significantly increased. So, is there an increased risk of major congenital abnormalities in infants when using GLP-1 receptor agonists or other non-insulin second-line antidiabetic drugs during pregnancy?
On December 11, 2023, researchers from institutions such as the Karolinska Institute, Harvard University, and Stanford University published a study titled “Safety of GLP-1 Receptor Agonists and Other Second-Line Antidiabetics in Early Pregnancy” in the JAMA Internal Medicine journal.
This is the first large-scale study investigating the potential risks associated with the use of non-insulin second-line antidiabetic drugs during human fetal development. The study reveals that women with type 2 diabetes using non-insulin second-line antidiabetic drugs (sulfonylureas, DPP-4 inhibitors, GLP-1 receptor agonists, or SGLT2 inhibitors) during pregnancy do not have a higher risk of major congenital abnormalities, including congenital heart defects, compared to those using insulin.

With type 2 diabetes becoming more prevalent in reproductive-age women and the recent approval of GLP-1 receptor agonists like semaglutide for obesity treatment, the number of pregnant women exposed to these drugs may increase.
The research team utilized health databases from Finland, Iceland, Norway, Sweden, the United States, and Israel, studying 3,514,865 pregnancy outcomes between 2009 and 2021. Among them, 51,826 cases (1.5%) involved women with type 2 diabetes before pregnancy, and 15,148 of them (29.2%) reported the use of first-line or second-line antidiabetic drugs during the periconceptional period (three months before pregnancy to three months after pregnancy).
The results show that the incidence of major congenital diseases, including heart defects, is significantly higher in infants born to mothers with type 2 diabetes compared to the general population.
Specifically, in the general population, the proportion of infants born with major congenital abnormalities is 3.76%, and the proportion with congenital heart defects is 1.31%. In women with type 2 diabetes, the proportions are 5.28% and 2.25%, respectively.
In the research population with type 2 diabetes, the proportions of infants born with major congenital abnormalities for those who did not use antidiabetic drugs during pregnancy or only used metformin were 4.77% and 5.32%, respectively. For those using insulin, the proportion was 7.83%, while for those using sulfonylureas, DPP-4 inhibitors, GLP-1 receptor agonists, or SGLT2 inhibitors, the proportions were 9.71%, 6.14%, 8.23%, and 7.04%, respectively. The proportions of infants born with major congenital abnormalities for those who did not use antidiabetic drugs during pregnancy or only used metformin were 2.30% and 2.04%, respectively. For those using insulin, the proportion was 4.20%, while for those using sulfonylureas, DPP-4 inhibitors, GLP-1 receptor agonists, or SGLT2 inhibitors, the proportions were 4.85%, 3.26%, 3.22%, and 3.88%, respectively.
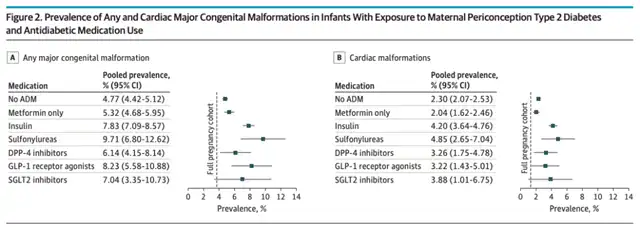
This data indicates that the risk of major congenital abnormalities, including congenital heart defects, in infants born to women with type 2 diabetes who use non-insulin second-line antidiabetic drugs (sulfonylureas, DPP-4 inhibitors, GLP-1 receptor agonists, or SGLT2 inhibitors) is not greater than those using insulin. The study provides initial safety assurance for infants exposed to non-insulin second-line antidiabetic drugs prenatally.
Paper Link: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2812743
Safety Study of GLP-1 Class Drugs During Pregnancy
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



