Targeting EGFR-Activated Myofibroblasts in Pancreatic Cancer Metastasis
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Cambridge Study: Targeting EGFR-Activated Myofibroblasts in Pancreatic Cancer Metastasis
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Cambridge Study: Targeting EGFR-Activated Myofibroblasts in Pancreatic Cancer Metastasis
“Targeting Candidate”! Cambridge University Paper Reveals New Mechanism for Pancreatic Cancer Metastasis.
Pancreatic ductal adenocarcinoma (PDAC) is associated with poor prognosis, and tumor-associated cancer-associated fibroblasts (CAFs) are recognized as potential therapeutic targets.
However, inadequate understanding of these heterogeneous cell populations hampers the development of effective treatment strategies.
On December 28th, researchers from the University of Cambridge published a study titled “EGFR-activated myofibroblasts promote metastasis of pancreatic cancer” in the journal “Cancer Cell.”
The researchers demonstrated that the epidermal growth factor receptor/Erb-B2 receptor (EGFR/ERBB2) signaling is induced by TGF-β through a dual-acting protein-mediated autocrine process in myofibroblasts (myCAFs).
In organ-derived cultures and mouse models of PDAC, inhibiting this EGFR/ERBB2 signaling network had varying effects on different subtypes of CAFs, providing insights into the mechanisms of their heterogeneity.
Finally, analysis of other cancer datasets suggested that these processes may occur in other malignant tumors. These findings shed light on the functional relevance of myCAF heterogeneity and identify potential targets for preventing PDAC tumor invasion.
(https://doi.org/10.1016/j.ccell.2023.12.002)
Research Background:
By 2030, pancreatic ductal adenocarcinoma (PDAC) is expected to become the second leading cause of cancer-related deaths. PDAC is often fatal because it is typically diagnosed after metastasis has occurred. Understanding the mechanisms of PDAC metastasis, prevention, and treatment is crucial. PDAC is unique among cancers due to its abundant non-malignant stroma, which promotes tumor growth and leads to drug resistance. This stroma is composed mainly of different types of tumor-associated fibroblasts (CAFs), including myofibroblast CAFs (myCAFs), inflammatory CAFs (iCAFs), and antigen-presenting CAFs (apCAFs), each with distinct molecular characteristics and potential functions.
Previous research identified interleukin-1 (IL-1) and transforming growth factor-beta (TGF-β) as major malignant cell-derived ligands inducing the formation of iCAFs and myCAFs, respectively. While downstream pathways of the IL-1 signaling pathway have been revealed as potential targets for iCAF treatment, the active pathways in TGF-β-induced myCAFs remain largely unknown.
Research Findings:
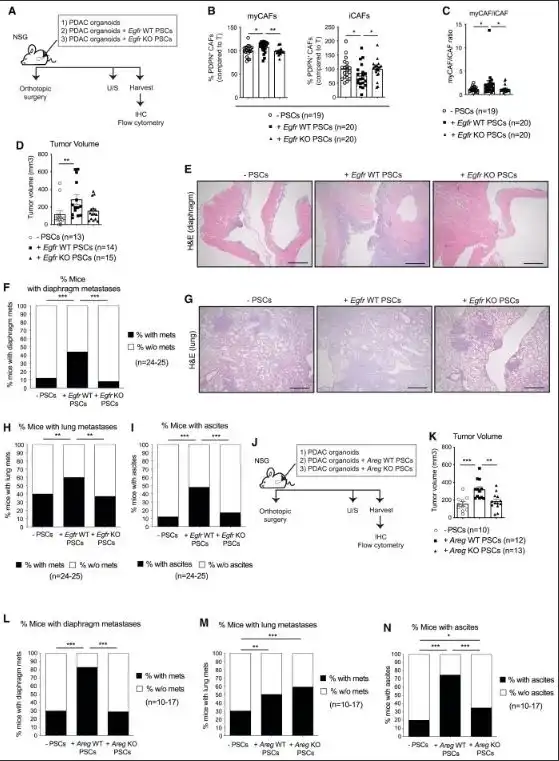
The researchers demonstrated the role of EGFR-activated myCAFs in promoting mouse PDAC metastasis. They unveiled the bidirectional crosstalk regulating PDAC myCAF molecular and functional heterogeneity, driving metastasis.
Observations suggested that other autocrine or paracrine mediators might help maintain continuous EGFR activation in myCAFs. Additionally, Amphiregulin (AREG) produced by malignant cells and/or immune cells, including macrophages, could further enhance EGFR/ERBB2 activation in myCAFs. The researchers validated a model: activation of the TGF-β pathway is essential for the induction of downstream AREG and effective EGFR signaling. Lastly, the researchers found that both EGFR and ERBB2 in pancreatic stellate cells (PSCs) were activated by TGF-β or PDAC-like organ conditioned media (CM) treatment. Inhibiting EGFR/ERBB2 not only significantly reduced AREG expression in myCAFs compared to just inhibiting EGFR but also impaired EGFR activation due to Erbb2 deletion. Thus, EGFR and ERBB2 were seen to cooperatively induce AREG expression and play a crucial role in TGF-beta-induced downstream signaling in myCAFs.
While the study identified the tumor-promoting role of EGFR-activated myCAFs, previous research suggested a tumor-suppressive role for alpha-smooth muscle actin (αSMA)-positive or Hedgehog (HH)-activated myofibroblasts, largely attributed to myCAF-mediated collagen deposition. Here, the researchers indicated that EGFR-activated CD90- myCAFs expressed lower levels of collagen compared to CD90+ myCAFs. Therefore, inhibiting EGFR/ERBB2 did not affect collagen abundance or overall fibrosis. In conclusion, these observations highlight the previously overlooked complexity of myCAFs in PDAC, requiring further understanding of their molecular and functional heterogeneity to devise effective combination strategies.
The study results indicate that genetic deletion or pharmacological inhibition of EGFR signaling in myCAFs impairs the malignant cell’s potential for metastasis by downregulating EMT signals, proven to promote PDAC malignant cell plasticity and metastasis in certain cases.
(https://doi.org/10.1016/j.ccell.2023.12.002)
The study suggests that inhibiting EGFR in PDAC may aid in combination with immunotherapy, benefiting cases with wild-type EGFR and reversing resistance to KRAS inhibitors.
These findings could have clinical significance for PDAC patients. Furthermore, the observations by the researchers may have broader implications, as AREG/EGFR signaling could be a common feature in many fibrotic diseases where myofibroblasts play a crucial role.
Research Conclusion:
This study reveals that the EGFR/ERBB2 signaling pathway is active in a subset of PDAC myCAFs, emphasizing the previously overlooked impact of EGFR/ERBB2 signaling inhibition on PDAC stroma, potentially extending to other malignant tumors.
The study identifies the role of EGFR-activated myCAFs in promoting PDAC metastasis.
Cambridge Study: Targeting EGFR-Activated Myofibroblasts in Pancreatic Cancer Metastasis
Reference: [https://doi.org/10.1016/j.ccell.2023.12.002](https://doi.org/10.1016/j.ccell.2023.12.002)
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



