Evidence of “Person-to-Person” Transmission of Alzheimer’s Disease Discovered!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Evidence of “Person-to-Person” Transmission of Alzheimer’s Disease Discovered!
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Evidence of “Person-to-Person” Transmission of Alzheimer’s Disease Discovered!
Autopsy Reports Suggest that Iatrogenic β-Amyloid Protein Could Cause Alzheimer’s Disease.
Can Alzheimer’s disease (AD) be transmitted from person to person? Don’t panic. Strictly speaking, this transmission is not the same as the transmission of the flu; AD is not an infectious disease.
However, scientists have indeed proposed a “pathogen” for AD—β-amyloid protein (Aβ).
On January 30, the journal “Nature Medicine” published new findings from the team led by John Collinge at University College London. This scholar can be considered an old friend, as they had previously discovered that Aβ in cadaver-derived human growth hormone (c-hGH) could induce Aβ deposition in mice, providing confirmation for the infectious hypothesis of AD.
This time, they analyzed 8 cases of individuals who had undergone c-hGH treatment during childhood. They found that some of these patients exhibited symptoms of early-onset AD and deposition of Aβ plaques in the brain. Surprisingly, these individuals did not carry the risk genes associated with early-onset AD.
This is undoubtedly strong evidence that iatrogenic Aβ can promote the progression of AD.

Proteins as pathogens may not be unfamiliar to many, as the stories of prions and mad cow disease have taught us a lot. Similarly, neurodegenerative diseases like AD, characterized by the accumulation of abnormal proteins, also have similar infectious hypotheses.
This story goes back to the last century.
As early as the 1950s, some people sought treatment for their height concerns. However, limited by the technology of that time, the growth hormone needed for treatment could only be extracted from the pituitary glands of deceased individuals, known as cadaver-derived human growth hormone (c-hGH). From 1959 to 1985, at least 1848 people in the UK underwent c-hGH treatment.
Unexpectedly, c-hGH caused another disease in some patients—Creutzfeldt-Jakob disease (CJD).
CJD is a neurodegenerative disease caused by misfolded PrP protein, the same prion responsible for mad cow disease. Prions can induce abnormal folding of normal PrP, leading to sponge-like holes in the brain. Worldwide, over 200 cases of iatrogenic CJD after c-hGH treatment have been identified, with over 80 cases in the UK.
Collinge’s team has been particularly interested in this issue. In 2015, they examined the bodies of 8 patients who died from CJD, all of whom had undergone c-hGH treatment in childhood. Surprisingly, besides the characteristic lesions of CJD, researchers found significant Aβ deposits and cerebral amyloid angiopathy (CAA) in the brains of 4 individuals.
These patients were aged between 36 and 52, quite young for AD. The probability of significant Aβ deposits in the brains of normal individuals at this age is less than 4%.
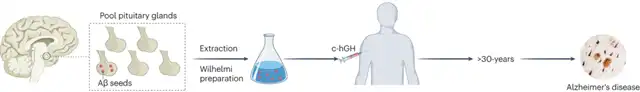
Fortunately, the c-hGH used by these patients was still available in the UK health department. Due to the preparation techniques at that time not being able to eliminate abnormal proteins from pituitary extracts, these c-hGH preparations contained prions causing CJD and Aβ related to AD.
In 2018, Collinge’s team published a paper in “Nature,” confirming that AD-related Aβ could induce Aβ deposition in the brains of normal mice like seeds. Surprisingly, c-hGH stored at room temperature for over 30 years still contained stable Aβ, capable of inducing Aβ deposition in the brains of mice.
Afterward, Collinge’s team continued to collect reports related to c-hGH, obtaining 8 cases with c-hGH treatment history between 2017 and 2022. These 8 patients did not have iatrogenic CJD, and 5 of them exhibited significant symptoms of early-onset dementia.
Among the 8 patients, the symptoms of patients 2/3/4/5/8 were consistent with early-onset dementia, showing progressive cognitive impairment in multiple areas, severely affecting daily life. The symptoms of patients 3/4/5/8 onset between 38 and 49 years, while patient 2 exhibited symptoms at 55.
Patients 3/4/8 have been diagnosed with AD, and 4/8 showed typical forgetfulness symptoms and met NIA-AA diagnostic criteria, while 3 exhibited non-forgetful language symptoms. The remaining two patients also met NIA-AA criteria for suspected AD. All 5 patients met DSM V criteria for major neurocognitive disorder due to AD.
Among the remaining 3 patients, patient 1 exhibited mild cognitive impairment symptoms according to NIA-AA definition at 42, patient 7 had subjective cognitive symptoms only, and patient 6 was asymptomatic.
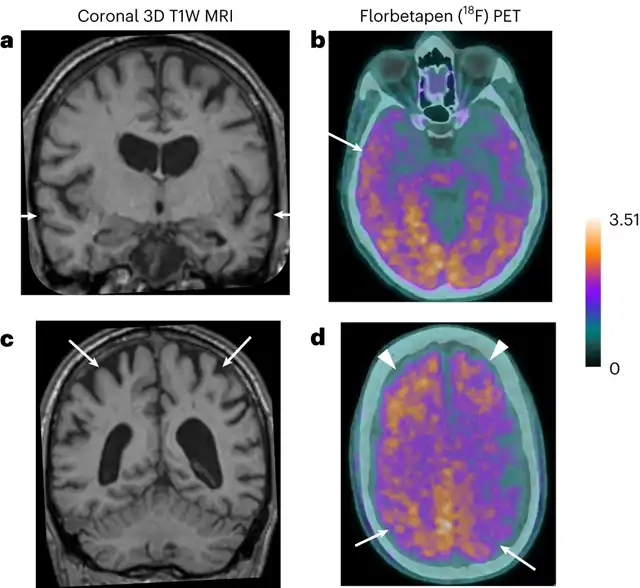
MRI and PET effects of Patient 3, showing significant bilateral temporal lobe atrophy
Patients 1/4/5 had no available samples, so the researchers performed genetic sequencing on the other 5 patients. Patient 2 was detected with a variant of uncertain significance in the amyloid precursor protein gene (app) (NM_000484.3: c.1486A>C; p.Lys496Gln), likely benign; only patient 2 carried the APOE4 allele. Other AD-related risk genes like ABCA7, SORL1, and TREM2 were not detected.
Although patients 1/4/5 had no genetic data, they did not have a family history of early-onset dementia or stroke (possibly related to CAA). It can be assumed that they are not carriers of familial AD genes.
In other words, although these patients showed symptoms consistent with early-onset AD, they did not carry genes associated with early-onset AD. Considering their young age, researchers believe that their AD symptoms are triggered by Aβ in c-hGH received during childhood, which is the commonality among the patients.
Interestingly, the presentation of these cases does not completely align with sporadic AD and familial AD. Researchers believe this may be an inherent feature of iatrogenic diseases.
This study only involves 8 samples, a small scale, and some scholars have questioned whether it can definitively prove that Aβ seeds drive the progression of AD. At least from a public health perspective, c-hGH treatment no longer exists, so there is no need to worry about related “infectious” issues. However, attention to iatrogenic infectious risks, such as those from surgical procedures, is still warranted.
Evidence of “Person-to-Person” Transmission of Alzheimer’s Disease Discovered!
References:
[1] https://www.nature.com/articles/s41591-023-02729-2
[2]https://www.nature.com/articles/d41586-024-00268-5
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



