What is the role of STAT proteins in cancer?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
What is the role of STAT proteins in cancer?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
What is the role of STAT proteins in cancer?
The signal transducer and activator of transcription (STAT) protein family includes STAT1, STAT2, STAT3, STAT4, STAT5 (STAT5A and STAT5B), and STAT6.
In cancer, STAT proteins, especially STAT3 and STAT5, act as signal transducers for cytokines, growth factors, and other molecules overexpressed in tumor cells, regulating the expression of numerous genes at the transcriptional and epigenetic levels.
These genes are involved in tumor cell proliferation, inhibition of cell apoptosis, promotion of cancer cell stemness, and drug resistance.
STAT proteins (especially STAT3) directly and indirectly regulate various metabolic processes in cancer cells, including aerobic glycolysis, oxidative phosphorylation, reactive oxygen species (ROS) production, mitochondrial energy generation, regulation of mitochondrial membrane integrity, glutamine metabolism, lipid synthesis, and lipid degradation metabolism.
STAT proteins also regulate the metabolism of immune cells in the tumor microenvironment, inducing immune suppression and tumor cell invasion.
Therefore, the metabolic reprogramming role of STAT proteins has a profound impact on tumor growth, anti-cancer immunity, and disease progression.
STAT proteins have become potential valuable targets for reversing metabolic dysregulation in various types of cancer.
In normal cells, ATP and precursors for biosynthesis are produced through glycolysis and oxidative metabolism pathways. Previous studies have found that even in the presence of oxygen, cancer cells switch to aerobic glycolysis instead of respiratory oxidative phosphorylation to support their rapid proliferation, a phenomenon known as the “Warburg effect.” Cancer cells can also utilize oxidative metabolism to produce ATP through the oxidation of fatty acids and amino acids in mitochondria. STAT proteins play important roles in aerobic glycolysis and respiratory oxidative metabolism in cancer cells.
STAT3, STAT5, and STAT6 regulate the expression and activity of glucose transporters and enzymes such as glucose transporter 1 (GLUT1) and GLUT3, hexokinase 2 (HK2), enolase 1 (ENO1), pyruvate kinase M2 (PKM2), and lactate dehydrogenase A (LDHA) in cancer cells, which are essential for glucose metabolism in cancer cells. In turn, PKM2 also promotes STAT3 and STAT5 signaling, with STAT5 activating hypoxia-inducible factor 1α (HIF1α), leading to further upregulation of glycolysis in cancer cells.
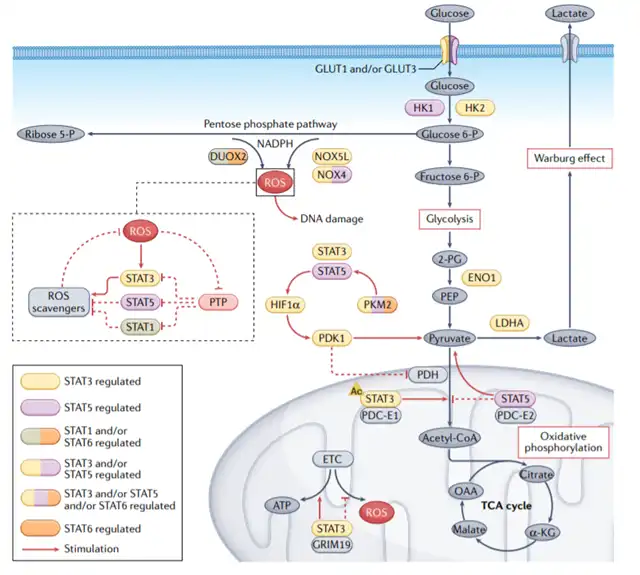
The pentose phosphate pathway is a branch of glycolysis that produces NADPH and 5-phosphoribosyl. NADPH oxidase (NOX) transfers electrons from NADPH to oxygen, producing ROS. Immune-regulating cytokines promote the upregulation of dual oxidase 2 (DUOX2), leading to the accumulation of ROS and DNA damage through the STAT1 and STAT6 signaling pathways. ROS activate Janus kinase 2 (JAK2–STAT3) signaling and STAT5, with STAT5 upregulating NOX4 and NOX5L to produce more ROS, enhancing the growth and proliferation of tumor cells.
In addition to regulating the Warburg effect, mitochondrial STAT3 also promotes oxidative phosphorylation and mitochondrial electron transport chain (ETC) activity. The interaction between STAT3 and the cell death regulator protein GRIM19 may promote the translocation of STAT3 to mitochondria. Once in the mitochondria, STAT3 interacts with ETC complexes I and II, leading to a decrease in ROS levels. Acetylation of STAT3 may also promote mitochondrial energy regulation by interacting with the pyruvate dehydrogenase complex (PDC) subunit E1 (PDC-E1). PDC is a mitochondrial enzyme that connects glycolysis and the tricarboxylic acid (TCA) cycle, and the binding of STAT3 to PDC accelerates the entry of pyruvate into the TCA cycle, further increasing ATP synthesis in cancer cells.
Furthermore, STAT5 promotes the Warburg effect by increasing the level of HIF1α, which drives the production of pyruvate dehydrogenase kinase isozyme 1 (PDK1), thereby preventing pyruvate from entering the TCA cycle and shifting metabolism to lactate production. Additionally, phosphorylated STAT5 interacts with PDC-E2 in mitochondria, activating STAT5-mediated transcription and promoting the transition of metabolism from oxidative phosphorylation to aerobic glycolysis.
Energy metabolism
Nicotinamide adenine dinucleotide (NAD+) is a coenzyme in oxidative phosphorylation redox reactions that is essential for energy metabolism. NAD+ levels decrease with human age and are associated with an increased incidence of cancer. Decreased NAD+ levels trigger IL-6 secretion, activating STAT3 signaling and promoting carcinogenesis and cancer cell stemness.
In some cancer cells, mitochondria are damaged, and ATP production is impaired. To compensate for energy deficiency, STAT3 induces the production of the malate-aspartate shuttle (MAS), which catalyzes the metabolic cycle that transfers hydride anions from NADH to NADP+, regenerating NAD+ and supplying NADPH. In melanoma cells carrying the BRAFV600E mutation, STAT5 induces the expression of nicotinamide phosphoribosyltransferase (NAMPT), which helps maintain NAD+ levels. Meanwhile, STAT5-induced NAMPT redirects melanoma cells towards an invasive phenotype associated with resistance to targeted therapy.
In addition to promoting NAD+ biosynthesis, the increased expression of NAMPT in cancer cells also increases the level of α-ketoglutarate (α-KG), leading to the upregulation of programmed cell death ligand 1 (PD-L1) through the STAT1-dependent IFN-γ pathway. Thus, the upregulation of NAMPT expression and NAD+ metabolism in cancer cells inhibits CD8+ T cell-dependent cytotoxicity.
Changes in lipid metabolism
Both lipid synthesis and lipid degradation metabolism play crucial roles in tumor initiation and treatment resistance. Cancer cells efficiently synthesize lipids and import lipids from the tumor microenvironment, responding to lipid factors, cytokines, and growth factors secreted by adipose tissue. STAT proteins not only increase lipid synthesis and degradation metabolism but also increase the lipid input of tumor cells, regulating cancer-promoting lipid metabolism at multiple levels.
STAT3 and STAT5 upregulate fatty acid binding proteins (FABPs) and CD36 (fatty acid transport proteins), leading to increased lipid accumulation, which supports the metastatic potential of tumor cells. HER2-mediated STAT3 activation transcriptionally upregulates fatty acid synthase (FASN), leading to increased de novo fatty acid synthesis. STAT5 also upregulates other enzymes important for lipid generation, including acetyl-CoA carboxylase 1 (ACC1) and sterol regulatory element-binding protein 1 (SREBP1). The metastatic potential of cancer cells is associated with increased phospholipid levels and decreased triglyceride levels.
Tumor necrosis factor-inducible protein 8-like protein 3 (TIPE3) also promotes the occurrence of lung cancer by activating STAT3, which is also associated with increased phosphatidylcholine levels and decreased triglyceride levels. STAT6 suppresses cholesterol synthesis through the miR-197–forkhead box protein J2 (FOXJ2) axis, thereby reducing endoplasmic ret
iculum stress in prostate cancer cells and promoting cancer cell proliferation.
Metabolic reprogramming is a hallmark of cancer, and understanding the role of STAT proteins in cancer metabolism is crucial for developing targeted therapies. Targeting STAT proteins may disrupt the metabolic symbiosis between cancer cells and the tumor microenvironment, offering new strategies for cancer treatment.
What is the role of STAT proteins in cancer?
references:
1.STAT proteins in cancer: orchestration of metabolism. Nat Rev Cancer.2023 Jan 3.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



