What is the role of Platelet Dynamics in Cancer Progression?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
What is the role of Platelet Dynamics in Cancer Progression?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
What is the role of Platelet Dynamics in Cancer Progression?
Platelets are the second most abundant type of blood cells after red blood cells.
They are short-lived cell fragments shed from mature megakaryocytes and have a lifespan of 7-10 days. Platelets are primarily known for their crucial role in hemostasis and pathological thrombus formation.
However, platelets also play a key role in other pathophysiological processes, including inflammation, tissue repair, and the growth and metastasis of tumors.
In recent years, platelets have been shown to interact dynamically with tumors, promoting the survival and proliferation of tumor cells.
Thrombocytosis in malignant tumor patients was first discovered in 1964.
Since then, increasing clinical evidence suggests a close relationship between elevated platelet counts in cancer patients and poor prognosis.
Numerous preclinical studies have further revealed direct and indirect crosstalk between platelets and tumor cells, sustaining tumor cell proliferation, metastasis, immune evasion, and chemotherapy resistance.
For example, platelets can bind to the surface of tumor cells to form microaggregates, establishing a physical barrier to protect tumor cells from immune cell attacks.
Platelets also release active soluble factors, such as TGF-β and platelet factor 4 (PF4), promoting tumor cell invasion and metastasis.
This intercellular communication stimulates tumor cells to secrete various cytokines, such as IL-6 and CCL5, inducing the production and secretion of additional platelets and various stimulatory factors promoting angiogenesis, metastasis, and tumor cell proliferation.
Therefore, a deeper understanding of the molecular mechanisms and dynamic roles of platelets in tumor progression and metastasis is essential.
This knowledge can aid in the development of their applications in diagnosis, prognosis, monitoring treatment response, and the development of precise antiplatelet strategies for safe and effective clinical cancer treatment.
Regulation of Platelets by Tumor Cells
In ovarian cancer, lung cancer, and gastric cancer, there is a higher incidence of thrombocytosis in patients with blood-borne metastasis and recurrence, especially in stage IV ovarian cancer, where the incidence of thrombocytosis can reach up to 65%. This suggests that an increase in platelet count may be a predictive factor for certain cancers and a method for monitoring tumor progression.
In ovarian cancer, tumor-induced thrombocytosis is mainly attributed to the production of IL-6 by tumor cells. IL-6 is released into the bloodstream, stimulating the production of thrombopoietin (TPO) in the liver.
Subsequently, TPO binds to TPO receptors (c-Mpl) on megakaryocytes, stimulating their growth and maturation in the bone marrow. Mature megakaryocytes then form platelet precursors that bud and eventually give rise to platelets.
Besides IL-6, tumor cells and stromal cells also secrete granulocyte-macrophage colony-stimulating factor (GM-CSF), CCL5, and PF4 into the tumor microenvironment, stimulating platelet production.
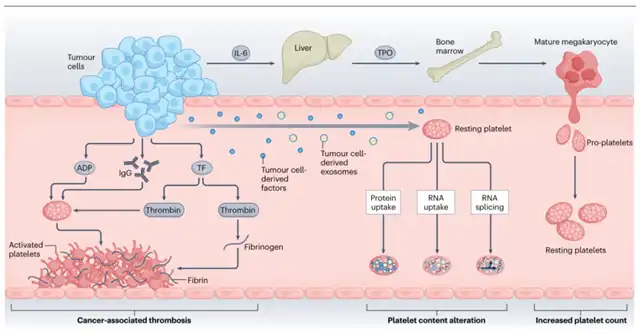
Studies indicate that factors secreted by tumor cells, such as adenosine diphosphate (ADP) and immunoglobulin G (IgG), or functional proteins and RNA, can lead to significant changes in the platelet proteome and transcriptome characteristics, enhancing platelet procoagulant, proangiogenic, and pro-metastatic properties. Circulating platelets can actively absorb tumor-derived proteins and mRNA, leading to increased levels of platelet-derived growth factor (PDGF), TGF-β, and matrix metalloproteinase 1 (MMP1).
Changes in platelet content and structure can induce platelet hyperactivity or activation. Platelet aggregation studies show that platelets from patients with metastatic ovarian cancer, pancreatic cancer, and breast cancer respond significantly more to agonists such as ADP, adrenaline, and collagen than platelets from healthy donors. Importantly, platelet activation may lead to cancer-associated thrombosis, which, if untreated, can result in thrombotic embolism and death. However, despite significant progress in understanding the interaction between platelets and tumor cells, little is known about the molecular mechanisms of how platelets respond to tumor signals to alter their activity or behavior.
Direct Interaction of Platelets with Tumor Cells
Direct contact between platelets and tumor cells triggers platelet activation and the formation of microaggregates around the surface of tumor cells, protecting them from immune recognition. Recent studies suggest that cancer cells in contact with platelets can engulf entire platelets through membrane fusion-dependent or dynamic-dependent phagocytosis. The platelet adhesion receptor CD42a can re-circulate to the cell membrane of A549 cells, which may contribute to the adhesion of cancer cells to the vascular wall in metastatic lesions.
Furthermore, in circulating prostate cancer cells isolated from patients, the absorption of platelets by these cells results in the transfer of lipids, RNA, and proteins from platelets to cancer cells, enhancing their stemness and proliferative capacity. These findings indicate that tumor cells can evade the immune system and enhance their proliferation and metastatic capabilities by absorbing, presenting, or utilizing platelet-derived lipids, nucleic acids, and proteins.
Platelets Support the Tumor Microenvironment
Platelets can also release many pro-survival, pro-angiogenic, and immune-regulatory factors in a non-contact manner, to build and maintain the primary and metastatic tumor microenvironment.
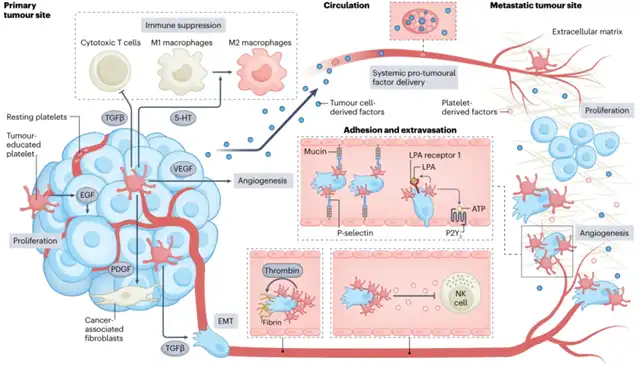
In primary tumors, platelets maintain tumor cell proliferation by secreting epidermal growth factor (EGF). Additionally, platelet-derived TGF-β and serotonin (5-HT) promote immune-suppressive microenvironments by inhibiting T cell function and promoting the M1-to-M2 macrophage phenotype transition. Platelets promote angiogenesis by secreting vascular endothelial growth factor (VEGF) and induce epithelial-mesenchymal transition (EMT) in tumor cells by secreting TGF-β. Platelet-derived PDGF can recruit cancer-associated fibroblasts, leading to excessive extracellular matrix deposition, hindering immune cell infiltration into tumors.
In circulation, platelet microaggregates can cover circulating tumor cells (CTCs) to protect them from shear stress and immune surveillance. During the formation of platelet microaggregates induced by tumor cells, thrombin-induced fibrinogen network formation stabilizes the binding of platelet microaggregates to the surface of CTCs. Additionally, sheddases ADAM10 and ADAM17 on platelets and TGF-β secreted by platelets inhibit the activity of natural killer (NK) cells.
At distant organ sites, platelets promote the adhesion of tumor cells to endothelial cells and contribute to the formation of solid metastatic foci by aiding the extravasation of tumor cells across the endothelium. Surface adhesion molecules on platelets, such as P-selectin, can bind to von Willebrand factor present on endothelial cells, facilitating the arrest of tumor cells at the endothelium. Platelets also release adenosine triphosphate (ATP) into circulation, interacting with P2Y2 receptors on endothelial cells to increase endothelial gaps, promoting the extravasation of CTCs. Meanwhile, platelet-derived lysophosphatidic acid (LPA) can bind to LPA receptor 1 on tumor cells, enhancing their invasive capabilities. Furthermore, circulating platelets can absorb tumor-derived factors and release them into the metastatic site, remodeling the pre-metastatic niche to facilitate the survival and growth of metastatic tumor cells.
Clinical Implications and Therapeutic Opportunities
Given the critical role of platelets in cancer progression and metastasis, targeting platelet-tumor interactions has emerged as a potential therapeutic strategy. Several preclinical and clinical studies are investigating the use of antiplatelet agents, such as aspirin, clopidogrel, and cilostazol, in cancer treatment. These drugs, traditionally used to prevent cardiovascular events, have shown anti-cancer effects by inhibiting platelet activation and interfering with platelet-tumor interactions.
Aspirin, a nonsteroidal anti-inflammatory drug (NSAID), is one of the most studied antiplatelet agents in the context of cancer. It inhibits cyclooxygenase (COX) activity, reducing the synthesis of pro-inflammatory prostaglandins. Aspirin also has antiplatelet effects, disrupting platelet activation and aggregation. Clinical trials, such as the Add-Aspirin trial, are evaluating the role of aspirin in preventing cancer recurrence in patients with resected early-stage cancer.
Clopidogrel, another antiplatelet drug, targets the P2Y12 receptor on platelets, inhibiting ADP-induced platelet activation and aggregation. Preclinical studies have suggested potential anti-cancer effects of clopidogrel, particularly in the context of colorectal cancer. Clinical trials are ongoing to evaluate the impact of clopidogrel in cancer patients.
Cilostazol, a phosphodiesterase 3 inhibitor, has antiplatelet and vasodilatory effects. Some studies have suggested its potential anti-cancer effects, and clinical trials are investigating its role in cancer treatment.
Beyond traditional antiplatelet agents, there is growing interest in developing more targeted therapies to disrupt specific platelet-tumor interactions. For example, targeting platelet receptors involved in adhesion to tumor cells, such as P-selectin and integrins, is being explored. Additionally, understanding the molecular mechanisms of platelet activation in response to tumor signals may unveil new therapeutic targets.
In conclusion (What is the role of Platelet Dynamics in Cancer Progression?)
Platelets play a multifaceted role in cancer progression, from supporting primary tumor growth to facilitating metastasis.
The dynamic interaction between platelets and tumor cells involves both direct physical contact and the release of soluble factors.
This interaction shapes the tumor microenvironment, promotes immune evasion, and contributes to the formation of metastatic foci.
Targeting platelet-tumor interactions holds promise as a therapeutic strategy in cancer treatment, and ongoing research is exploring the potential of antiplatelet agents and more targeted approaches in various cancer types.
As our understanding of the molecular mechanisms underlying platelet-tumor interactions deepens, new opportunities for precision medicine in cancer therapy may emerge.
What is the role of Platelet Dynamics in Cancer Progression?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



