RSV Vaccine Linked to Rare Neurological Disorder Risk!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
RSV Vaccine Linked to Rare Neurological Disorder Risk!
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
RSV Vaccine Linked to Rare Neurological Disorder Risk!
The U.S. FDA and CDC have warned that the Respiratory Syncytial Virus (RSV) vaccine may increase the risk of Guillain-Barré Syndrome (GBS), a rare autoimmune disorder, in older adults.
However, there is currently insufficient data to draw definitive conclusions. GBS is a rare autoimmune disease where the immune system attacks nerves, leading to symptoms such as muscle weakness and, in rare cases, paralysis.
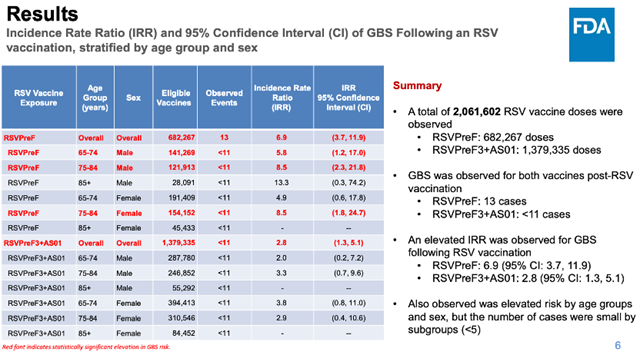
As of February 16, 2024, the CDC has identified 23 confirmed cases of GBS, all occurring within 22 days after receiving the RSV recombinant protein subunit vaccine.
Since the approval of these vaccines in the first half of 2023 in the U.S., Pfizer’s RSV vaccine has been administered to over 3 million people, while GSK’s RSV vaccine has been administered to over 6.5 million people.
Among the 23 reported GBS cases, 13 occurred in older adults who received Pfizer’s RSV vaccine, Abrysvo (RSVPreF), and no more than 11 cases occurred in older adults who received GSK’s RSV vaccine, Arexvy (RSVPreF3+AS01).
Within 21 days after vaccination, there were 25.1 cases of GBS per million doses of Pfizer’s RSV vaccine and 10.0 cases per million doses of GSK’s RSV vaccine.
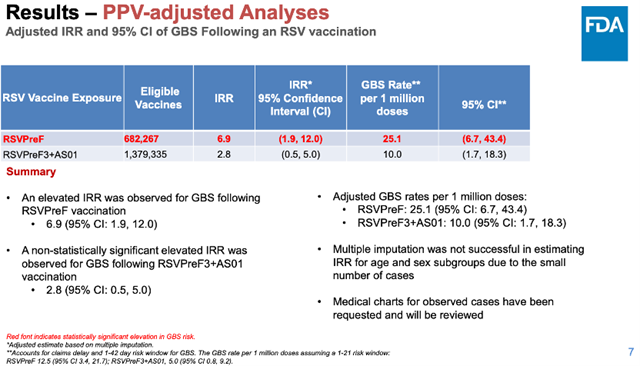
The Incidence Rate Ratio (IRR) of GBS with GSK’s RSV vaccine compared to unvaccinated individuals did not reach statistical significance, while the IRR of GBS with Pfizer’s RSV vaccine compared to unvaccinated individuals did reach statistical significance.
This suggests that, based on current data, Pfizer’s RSV vaccine increases the risk of GBS, but there is no evidence that GSK’s RSV vaccine increases the risk of GBS.
In addition to older adults, Pfizer’s Abrysvo has also been approved for use in pregnant women between 32 and 36 weeks of gestation to enhance immunity against RSV in infants.
Pfizer is continuing to develop Abrysvo for RSV prevention in children aged 2 to 17 and adults aged 18 to 60 through clinical trials.
There is a precedent for vaccines being withdrawn from the market due to rare diseases. In 1998, the first rotavirus vaccine was withdrawn from the market due to rare cases of intussusception.
If the increased risk of GBS from RSV vaccines is confirmed and regulatory agencies determine that the risk outweighs the benefits, it could lead to the withdrawal of market approval.
Both Pfizer’s and GSK’s RSV recombinant protein subunit vaccines are based on the RSV F protein. If the occurrence of GBS is related to the RSV F protein, it could challenge the safety profile of the RSV F protein principle.
RSV Vaccine Linked to Rare Neurological Disorder Risk!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



